Homework Help Overview
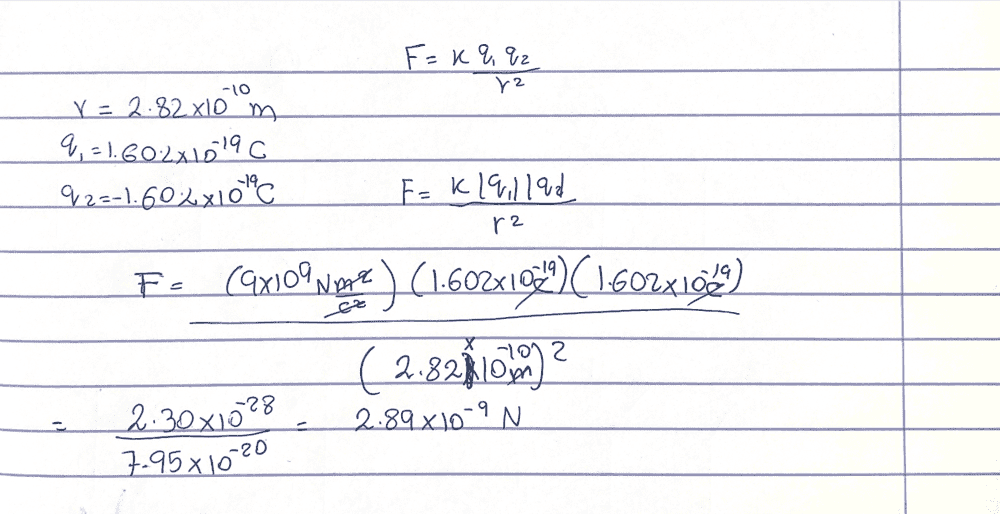
The discussion revolves around a problem related to Coulomb's Law, specifically focusing on the force of attraction between singly charged sodium and chloride ions in a salt crystal, with a given distance between them.
Discussion Character
- Exploratory, Assumption checking
Approaches and Questions Raised
- Participants are discussing the validity of calculations and the reliability of different answers found online. There is a suggestion to verify the calculations independently.
Discussion Status
The conversation is ongoing, with participants engaging in checking calculations and questioning the reliability of external sources. There is no explicit consensus on the correct approach or answer yet.
Contextual Notes
Participants note the potential for discrepancies in answers found online, highlighting the importance of verifying calculations independently.