HunterDX77M
- 42
- 0
Homework Statement
The following species has been discovered in interstellar space: HNC. Draw the Lewis Dot diagram.
Homework Equations
N/A
The Attempt at a Solution
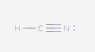
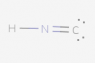
I tried several different configurations (see below), but they were all marked incorrect. Here is the basic method of Lewis Dot diagrams from my textbook:
1) Draw the skeletal structure with all the atoms, the least electronegative in the center. Hydrogens are used as terminal atoms.
2) Count the total number of valence electrons among all the atoms.
3) Begin by creating single bonds between the outer atoms and the central atom. Fill the octets of the outer atoms first.
4) If the central atom doesn't have a full octet, create double or triple bonds with the outer atoms to fill the octet.
So in total for this compound there are 10 valence electrons (1 from H, 5 from N and 4 from C). I've followed these rules, but still can't seem to get it.
Again, all the attached configurations were marked wrong. Please excuse the poor contrast (I have no control over that).