- #1
akoma714
- 9
- 0
One mole of an ideal diatomic gas at room temperature undergoes a transition from a to c along the diagonal path in Figure 19-23.
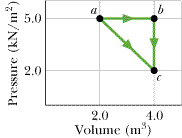
(a) During the transition, what is the change in internal energy of the gas?
-4987 J
(b) How much energy is added to the gas as heat?
____ J
(c) How much heat is required if the gas goes from a to c along the indirect path abc?
5013 J
I got part a and c right. I found the energies for path abc and added them together, because I couldn't find a way with path ac. Does the path I took matter for the heat? How do I find it using path ac?
(a) During the transition, what is the change in internal energy of the gas?
-4987 J
(b) How much energy is added to the gas as heat?
____ J
(c) How much heat is required if the gas goes from a to c along the indirect path abc?
5013 J
I got part a and c right. I found the energies for path abc and added them together, because I couldn't find a way with path ac. Does the path I took matter for the heat? How do I find it using path ac?