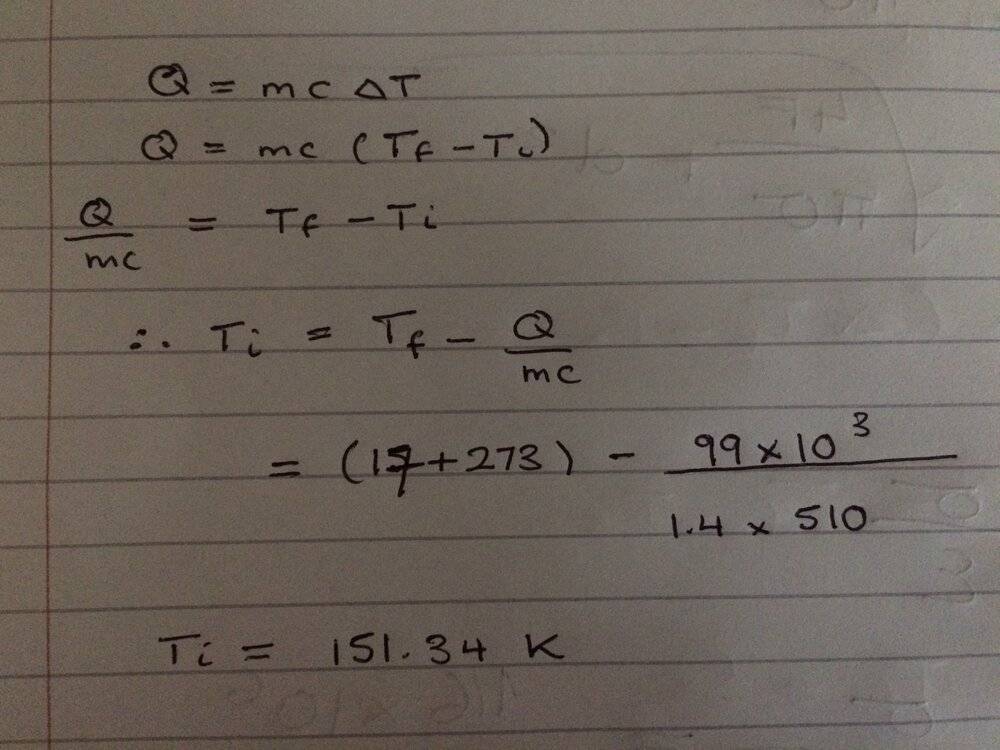Homework Help Overview
The discussion revolves around a problem involving heat dissipation by a steel disk, specifically focusing on the calculation of temperature changes related to heat energy loss. The subject area includes thermodynamics and heat transfer principles.
Discussion Character
Approaches and Questions Raised
- Participants explore unit conversions and the implications of negative heat energy values. Some express confusion about the problem statement and its physical relevance, while others analyze the mathematical setup and potential errors in calculations.
Discussion Status
There is ongoing dialogue about the interpretation of the problem and the calculations involved. Some participants have offered insights into unit handling and the mathematical approach, while others have raised concerns about the clarity of the problem statement and the assumptions made.
Contextual Notes
Participants note the ambiguity in the problem's wording and the potential for misinterpretation regarding the mathematical operations involved. There is also mention of the precision of numerical results in relation to the given data.