- #1
Amith2006
- 427
- 2
Sir,
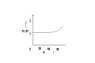
An experiment is carried on a fixed amount of gas at different temperatures and at high pressure such that it deviates from the ideal gas behaviour. The variation of PV/RT with P is shown in the following diagram. Is it right? Suppose a graph is plotted between PV/RT and T. At high temperatures, will the graph be identical to that between PV/RT and P i.e. will the value of PV/RT increase?
An experiment is carried on a fixed amount of gas at different temperatures and at high pressure such that it deviates from the ideal gas behaviour. The variation of PV/RT with P is shown in the following diagram. Is it right? Suppose a graph is plotted between PV/RT and T. At high temperatures, will the graph be identical to that between PV/RT and P i.e. will the value of PV/RT increase?