2Pac
- 37
- 0
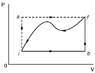
When a system is taken from state i to state f along the path iaf as shown in the figure, it is found that Qiaf = 53.6 cal and Wiaf = 30.5 cal. Along the path ibf, Qibf = 38.0 cal.
If the difference in volume between states i and b is 0.023 m3, what is the pressure dufference betwen states b and f?
If the difference in volume between states i and b is 0.023 m3, what is the pressure dufference betwen states b and f?