r12214001
- 24
- 2
- Homework Statement
- My calculation:
|210 X 101.3 – 45| = |-15 X (P)X 101.3 + 45|
- Relevant Equations
- N/A
Question:

Solution manual:
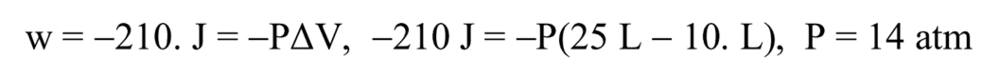
Is the solution manual correct or not? why does not contain heat transfer?
Solution manual:
Is the solution manual correct or not? why does not contain heat transfer?