confuted
- 28
- 0
Homework Statement
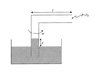
An open glass tube of uniform bore (uniform inner diameter) is bent into the shape of an "L". One arm is immersed into a liquid of density \rho and the other arm of length l remains in the air in a horizontal orientation. The tube is rotated with constant angular speed \omega about the axis of the vertical arm. Show that the height y to which the liquid rises in the vertical arm is
y=\frac{P_0}{g\rho}\left(1-exp\left[-\frac{\omega^2 l^2 M}{2RT}\right]\right)
where P_0 is the atmospheric pressure and M is the molecular weight of air and R is the universal gas constant. Assume that the air pressure inside open end of the tube is atmospheric pressure, as shown (see attached).Homework Equations
pV=nRT
\rho_{air}=\frac{m}{V}=\frac{MP_0}{RT} (from ideal gas law)
The Attempt at a Solution
I thought about trying to kludge something together with the centripetal acceleration causing a difference in pressure, but that didn't work out for me. Then I looked up Bernoulli's principle, and I don't see how I'm going to get an exponential out of that. What's the appropriate law here?
Attachments
Last edited: