proace360
- 27
- 0
Homework Statement
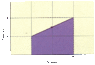
A certain amount of a monatomic ideal gas undergoes the process shown in the figure below, in which its pressure doubles and its volume triples.
In terms of the number of moles, n, the initial pressure, Pi, and the initial volume, Vi, determine the quantities tabulated below. Use n, Pi, and Vi to represent n, Pi, and Vi, respectively.
(a) work W done by the gas
(b) change in the internal energy U of the gas
(c) heat Q added to the gas
Homework Equations
W=integral of graph... lol
T=PV/nR
Change in Eint=(3/2)nRT
The Attempt at a Solution
ok i worked out (a) to be 3(Vi)(Pi) by finding the integral (area under the graph) and (b) to be (15/2)(Pi)(Vi) by finding the difference in temperatures and putting them in the energy equation (having the R value and n value cancel out). I'm fairly certain I am correct, but webassign says that they are wrong. The only reason I can think of why is because webassign treats the (Pi) as the greek Pi 3.14159 number, but I've been entering it the way it says.
Can someone confirm this with me/tell me why its treating it as pi?