- #1
lkh1986
- 99
- 0
Homework Statement
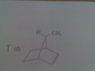
Does the molecule in the file attached have an enantiomer?
Homework Equations
The Attempt at a Solution
I think I can draw an internal symmetry plane to divide the molecule into 2 equivalent parts, but then again, it doesn't seem quite right to me. Building model helps, but I don't have the model with me now...