- #1
Icetray
- 84
- 0
[SOLVED] Thermodynamics - Pressure Question
A pressure cooker cooks a lot faster than an ordinary pan by maintaining a higher pressure and temperature inside. The lid of a pressure cooker is well sealed, and steam can escape only through and opening in the middle of the lid. A separate metal piece , the petcock, sits on top of this opening and prevents steam from escaping until the pressure force overcomes the weight of the petcock. The periodic escape of the steam in this manner prevents any potentially dangerous pressure buildup and keeps the pressure inside at a constant value. Determine the mass of the petcock of a pressure cooker whose operations pressure is 100 KPa gage and has an opening cross-sectional area of 4 mm^2 . assume an atomspheric pressure of 101 KPa, and a draw of the petcock
My Question:
I already have the solution to this question and it is stated that:
Atmospheric pressure is acting on all surfaces of the petcock, which balances itself out. Therefore, it can be disregarded in calculations if we use the gage pressure as the cooker pressure. A force balance on the petcock (ƩFy = 0) yields
Here is the diagram given:
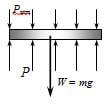
If the atmpospheric pressure is still pushing down on the petcock, how can we just ignore it?
Thanks in advance!
Homework Statement
A pressure cooker cooks a lot faster than an ordinary pan by maintaining a higher pressure and temperature inside. The lid of a pressure cooker is well sealed, and steam can escape only through and opening in the middle of the lid. A separate metal piece , the petcock, sits on top of this opening and prevents steam from escaping until the pressure force overcomes the weight of the petcock. The periodic escape of the steam in this manner prevents any potentially dangerous pressure buildup and keeps the pressure inside at a constant value. Determine the mass of the petcock of a pressure cooker whose operations pressure is 100 KPa gage and has an opening cross-sectional area of 4 mm^2 . assume an atomspheric pressure of 101 KPa, and a draw of the petcock
My Question:
I already have the solution to this question and it is stated that:
Atmospheric pressure is acting on all surfaces of the petcock, which balances itself out. Therefore, it can be disregarded in calculations if we use the gage pressure as the cooker pressure. A force balance on the petcock (ƩFy = 0) yields
Here is the diagram given:
If the atmpospheric pressure is still pushing down on the petcock, how can we just ignore it?
Thanks in advance!
Last edited: