You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Thermodyamics: Definition and 267 Discussions
No Wikipedia entry exists for this tag
-

Boltzmann vs Maxwell distribution?
So I worked through the Boltzmann distribution and got: $$ P\propto e^{\frac{-E}{k_BT}} $$ Where $E$ is the energy. So surely this means the kinetic energy (and therefore speed) of particles is distributed over a Boltzmann distribution. Or in equation: $$ P\propto e^{\frac{-mv^2}{2k_BT}} $$...- Toby_phys
- Thread
- Replies: 4
- Forum: Electromagnetism
-
J
Question about thermal conductivity in thermoelectrics
Does the phonon thermal conductivity account for a large part of the total thermal conductivity in thermoelectric materials like Bismuth Telluride? As far as I know, the phonon conductivity is the largest contributor to the total thermal conductivity in semiconductors. Is this still true for...- joebasqueo
- Thread
- Replies: 1
- Forum: Electromagnetism
-

Calculating Net Heat Flow for an Aluminum Disk in a Room
Homework Statement What is the net heat flow of an aluminum disk (emissivity = 0.05) with radius 10 cm and temperature 293K placed inside a room where the temperature is 300K? Asurface = π*r2 = 0.01π m2 Homework Equations Hnet = A*e*σ(Tradiate4-Tabsorb4) The Attempt at a Solution By simply...- lonelypancreas
- Thread
- Replies: 3
- Forum: Introductory Physics Homework Help
-
J
Temperature change (first law of thermodynamics)
Hi, From the first law of thermodynamics it follows: Cp * (δT/δt) = (δQ/δt) where Cp = specific heat capacity, T = temperature, Q = heat, t = time From this formula, you would derive that temperature keeps on increasing as long as dQ/dt > 0. But if you, for example, look at the idealized...- jones123
- Thread
- Replies: 4
- Forum: Thermodynamics
-
J
Thanks in advance.Heat Transfer Modeling: Get Insight for Physical Intuition
Hello everyone, I was hoping to get some insight on a model I am trying to create. Quick background in case it is important, I am now working at a new internship I landed for the summer doing some modeling and what not on areas of physics I have never worked on before. It has also been a few...- JordanD
- Thread
- Replies: 10
- Forum: Thermodynamics
-
E
Is work a function of state in adiabatic processes?
I'm reading a thermo book and I'm on the chapter dealing with the first law of thermodynamics. The book is discussing functions of state and how the internal energy is a function of state because it has a well defined value at every equilibrium state of the system. It says that the work and...- eprparadox
- Thread
- Replies: 1
- Forum: Mechanics
-

Relationship between HHV and LHV of gaseous propane
Homework Statement The Higher Heating Value (HHV) of gaseous propane is given as 2220.0 kJ/mol. What is the value of the Lower Heating Value (LHV) of gaseous propane? Data for water (all in kJ/mol): H(g, 25 deg. C) = 45.85 ; H(g, 100 deg. C) = 48.17 ; H(l, 25 deg. C) = 1.886 ; H(l, 100 deg. C)...- MickeyBlue
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
W
Problem involving thermal radiation and specific heat
Homework Statement A satellite to reflect radar is a 3.5-m-diameter, 2.0-mm-thick spherical copper shell. While orbiting the earth, the satellite absorbs sunlight and is warmed to 50 °C. When it passes into the Earth's shadow, the satellite radiates energy to deep space. You can assume a...- Wimpalot
- Thread
- Replies: 11
- Forum: Introductory Physics Homework Help
-
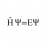
Ideal gas pressure from Maxwell-Boltzmann distribution
Hi everyone I'm having trouble with solving an exercise in statistical physics. I need to argue why the average number of particles with a velocity between ##v## and ##v+dv## that hit a surface area ##A## on the container wall in a time interval ##\Delta t## is $$N_{collision}=v_{x}A\Delta t...- H Psi equal E Psi
- Thread
- Replies: 4
- Forum: Electromagnetism
-
D
Exploring the Wonders of Physics: A High School Student's Journey
Hi guys! I'm a high school student and I love Physics. I hope this site will help me learn and understand a lot of (new) things! :)- Dranca Stefana
- Thread
- Replies: 1
- Forum: New Member Introductions
-

Schools Is a C grade in thermo really bad?
Hey all, I'm currently studying for my thermo final and it's really kicking my butt. My average has been a C and if I do EXTREMELY well on my final I'll be REALLY pushing for a B. I'm not sure how thermo is taught in other universities, but I'm learning it through statistical mechanics with...- Ahmed Abdalla
- Thread
- Replies: 7
- Forum: STEM Academic Advising
-

What is the Work Done by a Gas in a Cycle?
Homework Statement Homework Equations PV = nRT \\ W = - \int_{V_i}^{V_f} P dV \Delta E_{int} = Q + W The Attempt at a Solution a)[/B] Since this is a cyclic process, the change in internal energy of the system is 0. \Delta E_{int} = 0 The process causes some ice to melt, meaning heat...- knc
- Thread
- Replies: 24
- Forum: Introductory Physics Homework Help
-

Calculating work of Otto cycle stages - Thermodynamics.
One big coincidence of thermodynamics is that automobiles are usually powered by an Otto cycle This cycle consists of an adiabatic compression (the cylinder compresses), isochoric compression (the fuel ignites, increasing the temperature in too short a time for the piston to move), adiabatic...- Pull and Twist
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
D
Entropy of Vaporization Calculation for 5 Moles of Argon at 87.5 K and 150 K
Homework Statement 5 moles of liquid argon undergoes vaporization at its normal boiling point (87.5 K) and the resulting argon gas is subsequently heated to 150 K under constant volume conditions. Calculate the change of entropy for this process. The standard enthalpy change of vaporization, ∆...- dancingdodo27
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
D
Calculate the change in entropy for the system and the surroundings
Calculate the change in entropy for the system, the surroundings and the Universe if 2 moles of ethane are completely combusted at 298 K. Standard entropies of C2H5(g), O2(g), H2O(l) and CO2(g) are 229.6, 205.1, 69.9, 213.7 J mol-1 K-1 , respectively. Standard enthalpy changes of formation of...- dancingdodo27
- Thread
-
- Tags
- Change Entropy System Thermodyamics
- Replies: 13
- Forum: Biology and Chemistry Homework Help
-
E
Coefficient of performance of refrigerator
Homework Statement The pV-diagram in Fig. P20.51 (See attached file) shows the cycle for a refrigerator operating on 0.850 mol of H2. Assume that the gas can be treated as ideal. Process ab is isothermal. Find the coefficient of performance of this refrigerator. Homework Equations K = QC/|QH -...- Elias Waranoi
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
J
How to calculate heat dispersion through a solid copper rod?
I'm trying to build a fanless computer case for a small electronic device. I'm trying to figure out what type of heat-sink material I should use. I have a solid copper rod about 3cm in length with a radius of .05cm. I've determined and calculated the density, specific heat, thermal...- Jim Smith
- Thread
- Replies: 2
- Forum: Thermodynamics
-
T
Effect of Temperature on Chemical Reaction Equilibrium
here is the question (part I): For part I, I need some assistance, I cannot figure out how to do the question. I know eventually what to do, it's just working out the equilibrium constants I'm having trouble with. So to start, I want to work out the K values at 600K and 800K, I do this by...- Tom Hardy
- Thread
- Replies: 1
- Forum: Materials and Chemical Engineering
-
L
Cooling Steel Die for Contact Cooling of Aluminium - Advice Needed
Hi, I am designing a system to cool aluminium through contact cooling. I have a steel die with 9x 20mm diameter cooling chambers. Water is pumped through the chambers which cools the steel die and hopefully the aluminium sitting on top of it. I am looking to find the temperature of the...- Lewisjamesfc
- Thread
- Replies: 8
- Forum: General Engineering
-
R
Change in internal energy when water is heated from 0 to 4c
Homework Statement : [/B]Find the change in internal energy of 2kg water as it is heated from 0°C to 4°C. The specific heat capacity of water is 4200J/Kg and its densities at 0°C and 4°C are 999.9 kg/m3 and 1000kg/m3 respectively. Atm pressure=105PaHomework Equations :ΔU= Q-W W=PΔV M/V=D[/B]The...- randomgamernerd
- Thread
- Replies: 7
- Forum: Introductory Physics Homework Help
-
G
I Is a quasi-static but irreversible process possible?
Hello. I read the textbook of the thermodynamic and it said the definition of the reversible process as "thermodynamic process which is slow enough so the system state is always infinitesimally close to the thermodynamic equilibrium (quasi-static) during the process. Such a process can always...- goodphy
- Thread
- Replies: 5
- Forum: Classical Physics
-
D
Calculating Temp. in a Heated Vessel: A Problem for Dave
Hi there, I have a problem that I cannot find a solution too. In this problem I have a fuel (approximately natural gas) supply which is combusted when mixed with air inside a burner. The flame that is then generated is used to heat up a sealed cylindrical vessel (the flame is on the outside of...- davidgrant23
- Thread
- Replies: 1
- Forum: Thermodynamics
-
D
Integration of Thermodynamics equation w=–∫vDP
Homework Statement Integrate w=–∫vDP from 2 to 1 and get k(P2V2-P1V1/1-k) The equation is used for steady flow, reversible and Ideal gas Homework EquationsThe Attempt at a Solution I'm not sure how to get the result- Dmess2
- Thread
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help
-
F
Thermodynamics and thermometers (Thermistor)
1. The problem statement, all variables, and given/known data Q1 Resistance at 0 degrees Celsius = 3840 ohms Resistance at 100 degrees Celsius = 190 ohms Resistance at ? degrees Celsius = 2300 ohms a. Calculate the temperature of the water according to calibrated thermistor assuming the...- Faiq
- Thread
- Replies: 19
- Forum: Introductory Physics Homework Help
-
M
Thermodynamics: Compression of an Adiabatic Gas
Homework Statement Assume 1.500 mol of a monatomic ideal gas is compressed from 3.00 L to 1.00 L. a. If the initial and final temperature is 10.0 °C, what are the initial and final pressures (in atm)? b. How much work input (in kJ) is required if a reversible isothermal path at 10.0 °C is...- Minescrushessouls
- Thread
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
B
Calculating thickness of insulation, rate of T rise and fall
Homework Statement A.[/B] Someone is walking outside when the temperature of the air is -10°C. The metabolic rate of walking is 140 Cal/m2hr, and the usual muscle efficiency (20%) applies. They are completely covered by clothing with a coefficient of thermal conductivity of 0.36 Cal-cm/m2hr...- Brownstone
- Thread
- Replies: 6
- Forum: Introductory Physics Homework Help
-
R
Thermodynamics Change in Internal Energy?
Homework Statement A closed, rigid tank contains 2 kg of water, initially a two phase liquid–vapor mixture at T1 = 70°C. Heat transfer occurs until the tank contains only saturated vapor at T2 = 120C. Determine the heat transfer for the process, in kJ. answer choices: 3701kJ 119.4kJ 4835kJ...- Ricardeo Xavier
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-

Simplified Air-Conditioning Calculations
Hello everyone! I am currently having some real issues estimating the power requirements for a concept vehicle's Air-Conditioning System, and I mean I've been going over this on and off for the better part of a fortnight. At this stage I feel like I have fried my brain and have no idea if I am...- InfernoxCJC
- Thread
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help
-

Which Pressure Gauge Determines Saturation Temperature in a Closed Loop Pipe?
water is flowing in the closed looped pipe equipped with preheater. there are pressure and temperature gauges before the preheater and after the preheater. which pressure gauge should i use to know whether the outlet preheater temperature has reached the saturation temperature?- Sheroff
- Thread
- Replies: 1
- Forum: Mechanical Engineering
-

I Trying to understand the drinking bird toy better
I bought the drinking bird toy, and I want to get a better understanding of it, (https://en.wikipedia.org/wiki/Drinking_bird) I understand the basic way it works and that it is a simple heat engine, but I can't seem to find any information that helps me answer the following - I notice all...- Dr_Jekyll
- Thread
- Replies: 2
- Forum: Other Physics Topics
-
D
Thermodynamics - Need help with inclined plane problem
Homework Statement A train engine climbs a hill. The engine is 30% efficient. The train has a mass of 12000kg. The hill is 750m in height, with an incline of 20° form the horizontal. Friction exerts a force opposing motion of 8000N throughout the climb. Find w, Qin and Qout for the engine...- dlacombe13
- Thread
- Replies: 19
- Forum: Introductory Physics Homework Help
-
L
Thermodynamics Work from pressure
Homework Statement The tires on a bicycle require an air pressure of 80 psig. When isothermally pumped up the bicycle tires, the volume of the air (that was originally in the atmosphere) is reduced by a factor of 5.7. Please determine the work that must be done on each lbm of air that is pumped...- Logan McEntire
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-

Change in internal energy during water vaporization
According to the first law of thermodynamics, dQ = dU + dW and you can find dU = nCvdT If this is the case then when water at 100°C vaporizes to steam at 100°C shouldn't the change in internal energy be zero because it is dependent on temperature change?- Ian Baughman
- Thread
- Replies: 1
- Forum: Mechanics
-
F
Work done by particle in a box in expanding the box?
Homework Statement I'm given that the energy of a particle in a rectangular box is the following: E =\frac{\hbar \pi^2}{2m}(\frac{n_x^2}{L_x^2}+\frac{n_y^2}{L_y^2}+\frac{n_z^2}{L_z^2}) I'm to show that if the length of the box is increased adiabatically and quasistatically from L_x to 8L_x...- fordhamdining
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
G
What is the equilibrium temperature of a collector plate?
Homework Statement You would like to put a solar hot water system on your roof, but you're not sure it's feasible. A reference book on solar energy shows that the ground-level solar intensity in your city is 750 W/m^2 for at least 5 hours a day throughout most of the year. Assuming that a...- Galactium
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-

Thermodynamic Process: Is Work Conserved?
What is wrong with this logic, if any? It does not seem like this should be true but maybe I'm mistaken. Assuming the process consists of two isobaric processes and two isothermal processes the work from B to C in terms of p1, p2, V1, and V2 is given by the following. 1) WBC=p2(VC-VB) 2)...- Ian Baughman
- Thread
- Replies: 5
- Forum: Thermodynamics
-
Equilibrium temperature of some ice and steam
Homework Statement "A well-insulated bucket of negligible heat capacity contains 120 g of ice at 0°C. If 20 g of steam at 100°C is injected into the bucket, what is the final equilibrium temperature of the system?" Homework Equations $$Q_{fus} = m_{water}L_{fus}$$ $$Q_{vap} =...- ElPimiento
- Thread
- Replies: 9
- Forum: Introductory Physics Homework Help
-

Heat exchange in an isolated system
Homework Statement In a container of negligible mass, equal amounts (in weight) of ice at 0°C and steam at 100°C are mixed at atmospheric pressure. Assuming no heat exchange with the surroundings, what is the temperature when the system reaches equilibrium? What are the fractions of weights of...- Ian Baughman
- Thread
- Replies: 3
- Forum: Introductory Physics Homework Help
-
S
I Physics of Heat Transfer in Glass Windows
What is the physics behind heat transfer between two panes of glass? Commonly windows now are filled with argon (some cost) or krypton (pricey) At a given temperature all gas molecules have the same energy per mode, so heavy ideal gas molecules move more slowly than light ones, so heat...- Sherwood Botsford
- Thread
- Replies: 6
- Forum: Other Physics Topics
-
F
How to show that Carnot engine is more efficient?
Homework Statement Consider an Ideal gas engine with the following cycle: i. Isobaric expansion (T1 -> Th) ii. adiabatic expansion (Th -> T2) iii. Isobaric compression (T2 -> T_L) iv. adiabatic compression (T_L -> T1) a. Find its efficiency b. When operated in two temperature, show that Carnot...- flux!
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
I
Confusion: Internal energy u vs enthelpy h
I've been given the following relations where as I understand it subindex 2 equals subindex e and subindex 1 equals subindex i: ***EDIT*** More accurately subindex 1; initial state of a control mass subindex 2: end state of a control mass (end state is simply state 2 in the problem at hand)...- Imolopa
- Thread
- Replies: 7
- Forum: Thermodynamics
-
B
Why is this calculation of the enthelpy of combustion wrong?
Homework Statement A 1.250 g sample of ##C_8H_18## is burnt with excess oxygen in a bomb calorimeter. Change in temperature is from 294.05 K to 300.78 K. If heat capacity of the calorimeter is 8.93 kJ/K. Find the heat transferred to the calorimeter. Also calculate the enthalpy of combustion of...- Buffu
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
B
I Why triatomic gases have internal energy 7RT/2 ?
This table is given in my book, $$\begin{array}[c!c!c!c!] \text{ }&\text{ Transitional }&\text{ Rotational }& \text{ Vibrational} \\ \hline \text{Linear molecules} & 3&2& 3N -5\\ \hline \text{Non-Linear molecules} & 3&3& 3N -6\\ \hline \end{array}$$ It is also given...- Buffu
- Thread
- Replies: 14
- Forum: Atomic and Condensed Matter
-
V
A doubt from Kinetic Theory of Gases
I have read Average translation kinetic energy is 1/2RT per degree of freedom and Average translation kinetic energy for an ideal gases is 3/2RT.How? Does it imply f=3 for all ideal gases? -

Finding chemical potential with given thermodynamic relation
Homework Statement Suppose you are given the following relation among the entropy S, volume V , internal energy U, and number of particles N of a thermodynamic system, where A is a constant.: $$ S = A(NVU)^{\frac 1 3} $$ Find the chemical potential μ(T,P) Homework Equations $$ \frac μ T =...- Mayan Fung
- Thread
- Replies: 2
- Forum: Advanced Physics Homework Help
-
C
Entropy vs Enthalpy: Which is the Better Calculation for Heat Transfer?
When calculating heat transfer, how would one know when to use Q=T*m*(delta s) versus Q=m*(delta h). I'm confused when we should calculate using entropy versus enthalpy. Anything helps. Thank you!- causader90776
- Thread
- Replies: 1
- Forum: Mechanical Engineering
-
V
Understanding Heat Transfer in a Closed System with a Moving Piston
Homework Statement As shown in figure. Homework Equations dq=msdt[/B]The Attempt at a Solution I am unable to understand the question,what is the significance of the moving piston if the ends of the conducting rod is maintained at constant temperature.It is enough if someone could explain...- vijayram
- Thread
- Replies: 22
- Forum: Introductory Physics Homework Help
-
R
Heat Engine problem in Thermophysics
Homework Statement Im working on the following problem and could need some help in answering them: Work is being produced from a cycle. In order to produce this work, energy is being taking from a high temperature sources at a ratio of 1000 kJ/kg and the extra energy is being deliver to a...- reh
- Thread
- Replies: 5
- Forum: Introductory Physics Homework Help
-

Temperature dependence of Cv at very large volume
Homework Statement In the case of a gas obeying the equation of state $$\begin{align}\frac{Pv}{RT}&=1+\frac{B}{v}\end{align} $$ where ##B## is a function of ##T## only, show that, $$\begin{align}c_v&=-\frac{RT}{v}\frac{d^2}{dT^2} (BT)+\left(c_v\right)_0\end{align}$$ where ##\left(c_v\right)_0##...- arpon
- Thread
- Replies: 10
- Forum: Advanced Physics Homework Help
-

I Can internal energy be calculated from equation of state?
We know, $$dU=TdS-PdV$$ ##\int PdV## can be calculated if the equation of state is given. I tried to express ##S## as a function of ##P ,V## or ##T## (any two of those). $$dS=\left(\frac{\partial S}{\partial V}\right)_T dV+\left(\frac{\partial S}{\partial T}\right)_V dT$$ $$=\left(\frac{\partial...- arpon
- Thread
- Replies: 2
- Forum: Other Physics Topics