Percent Definition and 224 Threads
-
R
How Do You Calculate Weight Percent Concentration of KCl in Water?
Homework Statement What is the weight percent concentration of a saline solution prepared by dissolving 2.440 mole of KCl in 10 L of water? Homework Equations The Attempt at a Solution- rridl001
- Thread
- Concentration Percent Weight
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
U
Partial derivative of function w.r.t. the percent change of the variable
Homework Statement Rewrite this in terms of f, f, ∂f/∂x, and x: ∂f(x,y)/∂(%Δx) = ∂f(x,y)/∂(d log(x) ) Homework Equations ∂(%Δf(x,y))/∂(%Δx) = ∂logf(x,y)/∂log(x)= ∂f(x,y)/∂x*x/f(x,y). ∂f(x,y)/∂log(x)=x∂f(x,y)/∂x The Attempt at a Solution I found that (%Δx) can be written as...- Usuiisu
- Thread
- Change Derivative Function Partial Partial derivative Percent Variable
- Replies: 2
- Forum: Calculus and Beyond Homework Help
-
K
Calculating % Yield of Ester in Chemistry Lab
% yield ester I'm having a problem with my chemistry lab report. the question is: What would the % yield of ester be if the reaction were simply allowed to equilibrate (i.e. no products are removed)? I think the the yield is going to smaller but i don't know why. pls help- kosterot
- Thread
- Percent Percent yield Yield
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
M
Centripetal Force Lab Percent Error and Accepted Values Help PLEASE
Hi! I need help and please do not delete this! What I need help with i cannot put into the template provided. I am sorry but I need help with a lab report. PLEASE I AM BEGGING YOU TO NOT DELETE THIS BECAUSE IT DOES NOT FOLLOW THE TEMPLATE! I REALLY REALLY NEED HELP! I need to find the...- mickchick1
- Thread
- Centripetal Centripetal force Error Force Lab Percent
- Replies: 5
- Forum: Introductory Physics Homework Help
-
B
Percent change in thermionic emission
% change in thermionic emission Q1. Determine the % change in thermionic emission for an oxide-coated filament of work function of 1.3eV if the temperature is decreased by 1.00% at a temperature of 2300K I'm uncertain but i used dJ/J=dT/T ( 2 + ((1160x1.3)/1000) to get a 15% change in...- benben312000
- Thread
- Change Emission Percent Thermionic emission
- Replies: 3
- Forum: Engineering and Comp Sci Homework Help
-
M
Partial derivatives and percent error
Homework Statement When two resistors R1 and R2 are connected in parallel, their effective resistance R = (R1R2)/(R1+R2). Show that is R1 and R2 are both increased by a small percentage c, then the percentage increase of R is also c. Homework Equations The Attempt at a Solution I...- MeMoses
- Thread
- Derivatives Error Partial Partial derivatives Percent
- Replies: 1
- Forum: Calculus and Beyond Homework Help
-
J
Maximum Percent Recovery of pure acetanilide?
Homework Statement Solubility of acetanilide in water is 5.5g/100mL at 100C 0.53g/100mL at 0C A 0.150g sample of acetanilide containing only traces of impurities was recrystalized using a total of 3mL of hot water. a) What is the maximum possible percent recovery of pure acetanilide...- JameB
- Thread
- Maximum Percent Pure
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
B
Percent of initial total mechanical energy lost during a round trip?
Given: Mass= 5 kg Vo= 40 m/s Vf=50 m/s s=150 m Ho=0 Hf=50.2 m g=9.8 m/s/s friction=6.26 N θ=30° *You will probably not use all of these values* What percent of initial total mechanical energy is lost during the round trip? (a block (with mass m) going up an inclined plane, and...- bmx_Freestyle
- Thread
- Energy Initial Lost Mechanical Mechanical energy Percent
- Replies: 7
- Forum: Introductory Physics Homework Help
-
M
Percent error using acceleration and 1/mass
I'm doing a lab where I had to use a cart and a pully, collecting data to compare the net force and the acceleration and again for acceleration vs mass. The acceleration vs mass graph was a curve, which we then straightened out to give us a graph of acceleration vs 1/mass. I am wondering how i...- mjohnston2
- Thread
- Acceleration Error Percent
- Replies: 2
- Forum: Introductory Physics Homework Help
-
J
Rotating Polarizer 90 Degrees With Only 10 Percent Loss
Homework Statement Describe how to rotate the plane of polarization of a plane-polarized beam of light by $90^\circ$ and produce only a 10 percent loss in intensity using "perfect" polarizers. The solution says to use 24 polarizers, with each at an angle of $3.75^\circ$ with the next...- JSGandora
- Thread
- Degrees Loss Percent Polarizer Rotating
- Replies: 1
- Forum: Introductory Physics Homework Help
-

Chemistry Find Mole % of N2H4 in Mixture: Homework Statement
Homework Statement A mixture of NH3 and N2H4 is placed in a sealed container at 300K. The total pressure is 0.5atm. The container is heated to 1200K, when both substances decompose completely according to the equations, 2NH3--->N2+3H2 N2H4--->N2+2H2 After decomposition is complete, the...- Saitama
- Thread
- Mole Percent
- Replies: 8
- Forum: Biology and Chemistry Homework Help
-
S
Calculating percent abundance for 3 isotopes
For my chemistry class, I need to be able to calculate percent abundances for multiples isotopes, if given the mass of the isotopes and average atomic mass of the element. The percent abundance of 1 isotope may be given. The teacher has said that calculating for a problem with 3 isotopes is... -
G
Producing Phosphoric Acid: Calculating Percent Yield
Approximately 12 million kilograms of phosphoric acid (H3PO4) are produced annually for fertilizers, detergents, and agents for water treatment. Phosphoric acid can be prepared by heating the mineral fluoroapatite (Ca5(PO4)3F) with sulfuric acid in the presence of water. Ca5(PO4)3F + 5H2SO4 +...- googol
- Thread
- Acid Percent Percent yield Yield
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
L
Percent Error Formula: Find the % Error in Lab w/ 10 mL Water
Homework Statement I need to find the percent error from a lab I did in class. i have all the data but I don't know what would be used for the theoretical weight and experimental weight in the percent error formula. I used 10 mL, 25 mL, and 50 mL of water in a graduated cylinder. I'll just...- lindseyam
- Thread
- Error Formula Percent
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
I
Percent change in photon energy
[b]1. Homework Statement A photon of wavelength is incident on an electron at rest. On collision, the photon is scattered at an angle θ with increased wavelength . Suppose θ = pi/2, what is the percent change in photon energy for this collision in (a) a microwave oven with wavelength = 3.0...- ikstellub
- Thread
- Change Energy Percent Photon
- Replies: 1
- Forum: Introductory Physics Homework Help
-
U
What Happens to Percent Error as the Number of Vectors Increases?
Homework Statement What will happen to the percent error of graphical or force table method as the number of vectors to be added increases? Give proof. 3 vectors A = 400 g B = 450 g C = 427 g Homework Equations The Attempt at a Solution First, I computed for the percent...- un_cooled
- Thread
- Error Percent Vectors
- Replies: 1
- Forum: Introductory Physics Homework Help
-
G
Compute percent uncertainty of Resistor
Homework Statement The relationship between resistance with temperature is expressed by the equation of R= R0[1+ α(T-T0)], where Ro is the resistance at the reference temp. T0 and α for the resistor material has been determined to be 0.0048 ± 0.1% oC. In the range 0 to 100* C, in which we...- g.sharm89
- Thread
- Percent Resistor Uncertainty
- Replies: 1
- Forum: Calculus and Beyond Homework Help
-
J
Matlab % Error for Euler's Method
Homework Statement I am trying to find the percent error in Euler's method with 3 different step sizes. The Attempt at a Solution Below is some coding I have calculating percent error of Euler's method however there has to be a more efficient way to input the matrices, I have found...- John31
- Thread
- Error Matlab Percent
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help
-

What percent of professors have open positions for new grad students?
It seems that most professors seem to have positions open for undergrads (although undergrads don't need pay). But what about graduate students? Is it a good idea to *always* email any prospective advisor, asking him if he has open positions available for new grad students? It seems that...- Simfish
- Thread
- Grad Percent Professors students
- Replies: 6
- Forum: STEM Academic Advising
-
P
Proof of 50% Rule in Memory Fragmentation Analysis
statistical analysis of first fit for instance reveals that even with some optimization given N allocated blocks, another 0.5N blocks will be lost to fragmentation. that is one-third of memory may be unusable. This property is known as the 50-percent rule. can anybody explain to me the proof...- prashantgolu
- Thread
- Percent
- Replies: 2
- Forum: Programming and Computer Science
-
G
Percent water vapor - greenhouse gas
I'm have armchair interest in greenhouse gases. One of the interesting points is the amount of greenhouse effect that CO2 has versus water vapor. I go to this page http://www.climate4you.com/GreenhouseGasses.htm and find that the relative percentage of atmospheric humidity is 45%. I...- glenncz
- Thread
- Gas Greenhouse Greenhouse gas Percent Vapor Water Water vapor
- Replies: 7
- Forum: Earth Sciences
-
N
Percent Difference Between Theta and Sin (Theta) help
Homework Statement This is what my professor is asking me to do: Show a table for when sin(theta) and theta have a percent difference of 1%, 5%, and 10%. (i.e. this table will have three columns)" You are to show this table (for all three quantities) such that the behavior leading slightly...- Nomad5133
- Thread
- Difference Percent Sin Theta
- Replies: 22
- Forum: Calculus and Beyond Homework Help
-
L
Calculate the percent difference of free fall acceleration.
1. Calculate the percent difference of freefall acceleration for the light and heavier picket fence Lighter Picket Fence - Mass 95.84 g | Acceleration 6.4141 m/s2 Heavier Picket Fence - Mass 128.16 g | 8.995 m/s2 2. Compare each of the values from the graphs to the accepted value of free...- lokobreed
- Thread
- Acceleration Difference Fall Free fall Percent
- Replies: 1
- Forum: Introductory Physics Homework Help
-
G
Calculating Resultants and Percent Error
Well I am almost done with my lab report finally and I just hit a speed bump. When I calculate my percent error something popped up in my calculator showing that I have like 80% error in the last 2 problems. Anyone know why am I getting this huge marginal error? please note that 30 125 200 288...- godkills
- Thread
- Error Percent
- Replies: 1
- Forum: Introductory Physics Homework Help
-
M
Understanding the true impact of gun violence: A scientific perspective
Hi, I'm not sure if I'm posting this in the correct place or not. I'm having a discussion with one of my high school age daughters about gun violence. We've started this discussion because of the recent shooting in Arizona. I'm trying to explain to her that given the number of guns in the US...- mikeR3975
- Thread
- Percent
- Replies: 12
- Forum: Precalculus Mathematics Homework Help
-
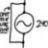
Computing true percent relative error (Taylor Series)
Homework Statement See figure attached, Homework Equations The Attempt at a Solution Isn't the Maclaurin series just simply the Taylor series around 0? (\text{i.e. } (x-c), \quad c=0, \quad x ) Also for part B, how do we go about solving for | \epsilon_{t} |? Thanks...- jegues
- Thread
- Computing Error Percent Relative Series Taylor series
- Replies: 1
- Forum: Calculus and Beyond Homework Help
-
P
What Is the Percent Yield of Al2O3 in This Reaction?
Homework Statement If the reaction of 2.5 g of Al with 2.5 g of O2 produced 3.5 g of Al2O3, what is the percent yield of Al2)3? Be sure to write and balance equation. Homework Equations Percent Yield = (actual yield / theoretical yield ) x 100 The Attempt at a Solution 4Al + 302...- priscilla89
- Thread
- Percent Percent yield Yield
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
P
Percent Composition of Carbon in Dimethylsulfoxide (C2H6SO4)
Homework Statement What is the percentage of carbon in dimethylsulfoxide, C2 H6 SO4? Homework Equations mass of C / total mass x 100 = percentage of carbon The Attempt at a Solution The total mass = 126 total mass of C = 24 Wouldn't it be 24 / 126 x 100 = 19 % But...- priscilla89
- Thread
- Composition Percent
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
3
Percent Growth of Exponential Function
Homework Statement If there is a quantity T comprised of two other quantities such that T=L+B, and quantity T and B are both increasing in every period at a fixed percent such that %growth T > %growth B, it will be true that %growth L > %growth T. It will also be true that as n approaches...- 3.141592654
- Thread
- Exponential Exponential function Function Growth Percent
- Replies: 1
- Forum: Precalculus Mathematics Homework Help
-
K
Calculate Mass Percent: HNO3 in Sample
Homework Statement A sample conaining both HCl and HNO3 had a density of 1.083g/ml. A 43.00 ml amount of this sample required 29.09 ml of 0.2828 M NaOH for titration. Another 43.00 ml amount of this sample required 7.88 ml of 0.1505 M AgNO3 for titration. Calculate the mass percent of HNO3...- Ki-nana18
- Thread
- Mass Percent
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
M
Percent difference and uncertainty
Homework Statement How do we solve this problem? % Difference = (l 0.282 +/- 0.0019 - 0.288 +/- 0.04 l) / 0.285 +/- 0.02 Homework Equations Percent Difference = (l value - average value l)...- mike2601
- Thread
- Difference Percent Uncertainty
- Replies: 1
- Forum: Introductory Physics Homework Help
-
L
Volume of Sulphuric Acid (Mass Percent)
The Density of a solution of sulphuric acid is 1.8022g/cc, and it is 88.00 percent by mass. What volume of the solution (in milliliters) do you need to supply 43.0 g of sulphuric acid? I'm not too sure how to solve this question, I tried it and thought it was too simple? This is what I did...- lily_moss14
- Thread
- Acid Percent Volume
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
C
What Is the Mass Percent of Iron in the Compound?
Homework Statement 0.3106 g of an iron-containing compound yield 0.07017 g Fe2O3 upon oxidation. What is the mass percent of the iron in the compound?? Homework Equations stoichiometry? The Attempt at a Solution (0.07017 g Fe203)(1 mole Fe2O3/ 159.7 g Fe2O3)(2 moles Fe/1 mole...- Complexity
- Thread
- Chemistry Mass Percent
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
N
Molar Solutions with percent concentrations
1. The molecular weight of NaCl is 58.54. A 1 M solution of NaCl has 58.54 g NaCl dissolved in distilled water to a total volume of 1000 mL or 1 L. a .1 M solution has .854 g/L. A .2 M solution has 11.71 g/L. Qustion 1 - What is the molarity of a 5% w/v NaCl solution? Question 2 - What is...- NoobSandwich
- Thread
- Percent
- Replies: 9
- Forum: Biology and Chemistry Homework Help
-
N
How Do You Calculate the Percent by Mass of Na2SO4 * 10H2O in a Hydrated Sample?
Homework Statement A thoroughly dried 1.283 sample of Na2SO4 is exposed to the atmosphere and found to gain 0.395 in mass. What is the percent, by mass, of Na2SO4 * 10H20 in the resulting mixture of anhydrous and the decahydrate? Homework Equations 1 mol Na2SO4 = 142.0428 g 1 mol H20...- Nano
- Thread
- Chemistry Mass Percent
- Replies: 9
- Forum: Biology and Chemistry Homework Help
-
C
Percent change in mass when gaining charge.
Homework Statement When an object such as a plastic comb is charged by rubbing it with a cloth, the net charge is typically a few microcoulombs. If that charge is 3.6*10^-6 C, by what percentage does the mass of a 39g comb change during charging? Homework Equations n/a? The...- Cllzzrd
- Thread
- Change Charge Mass Percent
- Replies: 7
- Forum: Introductory Physics Homework Help
-
M
Calculating Error Rate for Valid Results: A Wins Over 99.9% of the Time
there are 1000 valid results, but 1 is invalid. in other words, 99.9% of the results are valid. what is the error rate for this to be possible? basically right now i have these facts -- if A has more than B, A wins. in order for a result to be considered "valid," A must have more wins than B...- magnifik
- Thread
- Error Percent
- Replies: 5
- Forum: Engineering and Comp Sci Homework Help
-
P
Redox titration (calculating percent iron in sample)
Homework Statement A mass of iron ore weighing 0.2792g was dissolved in dilute acid and all the iron was converted to Fe2+(aq). The iron II solution required 23.30ml of 0.0194M KMnO4 for titration. Calculate the percentage of iron in the ore.Homework Equations Wrote out my redox reactions...- philtered
- Thread
- Iron Percent Redox Titration
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
W
Calculating percent error in a lab
calculating percent error in a lab please help! Homework Statement I have to calculate an experimental value for g using values from my lab. I got -45.9 as my value, and now i have to calculate the percent error Homework Equations a= g (m2 - m1/ m2+m1) was the equation i used...- wiccabean21
- Thread
- Error Lab Percent
- Replies: 1
- Forum: Introductory Physics Homework Help
-
0
Calculate Mass Percent of Cu & Zn in Brass Alloy
Brass, an alloy of Zn and Cu, reacts with hydrochloric acid as follows: Zn (s) + 2 HCl (aq) → ZnCl2 (aq) + H2 (g) Cu does not react with HCl. When 0.5065 g of a certain brass alloy is reacted with excess HCl, 0.0985 g of ZnCl2 is eventually isolated. What is the composition of the...- 00_Furon
- Thread
- Mass Percent
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
G
Solve Ka: Percent Ionization of Formic Acid - 2.85%
Homework Statement What concentration of formic acid would give an aqueous solution in which 2.85 percent of the formic acid molecules are ionized? Assume that Ka for formic acid is 1.80E-4 . Homework Equations The Attempt at a Solution X^2 / .0285 - X = 1.8E-4 X = .00226 M =...- George3
- Thread
- Ionization Percent
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
R
Percent increase/decrease cylinder (no values)
By approximately what percent would the volume of a right circular cylinder increase or decrease if the height were to increase by 2% and the radius of the base were to decrease by 1.5%? Partial differentials makes sense in this case, I am just stuck when I have no usable values. Volume of...- riskybeats
- Thread
- Cylinder Percent
- Replies: 3
- Forum: Calculus and Beyond Homework Help
-
J
Percent change in tension to achieve specified change in frequency
% change in tension to achieve specified change in frequency Homework Statement A particular guitar string is supposed to vibrate at 219 Hz, but it is measured to actually vibrate at 224 Hz. By what percentage should the tension in the string be changed to get the frequency to the correct...- Jennifer_ea
- Thread
- Change Frequency Percent Tension
- Replies: 2
- Forum: Introductory Physics Homework Help
-
M
Determining the Mass percent comp. of an Aqueous Hydrogen peroxide
Homework Statement In lab we recorded a barometric pressure of 768.2 torr. Suppose we didn't have access to a barometer and assumed that atmospheric pressure was 760 mm Hg. Would the mass percent of hydrogen peroxide that you calculated for the solution be higher or lower than the value you...- Marie123
- Thread
- Aqueous Hydrogen Hydrogen peroxide Mass Percent
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
S
Weight percent of hypochlorite in bleach
Homework Statement If 5 mL of a bleach sample is diluted to 100 mL and then 30.00 mL of the dilute solution is titrated with 29.65 mL of a 0.1034 M solution of thiosulfate what is the weight percent of hypochlorite in the 5 mL of bleach? Homework Equations OCl−(aq) + 2I−(aq) → I2(aq) +...- ~Sam~
- Thread
- Percent Weight
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
C
What Is the Maximum Percent Recovery Using Water for Recrystallization?
One gram of salicylic acid dissolves in 460 mL of water at room temperature and 15 mL of boiling water. What is the maximum percent recovery that can be achieved using water as a recrystallization solvent assuming the recrystallization mixture is not cooled below room temp.? So I assumed we...- ChemIsHard
- Thread
- Maximum Percent
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
H
Percent by weight of substance in hydrate
Q: Gypsum, used for wallboard, has the formula CaSO4 2H2O. (a) What is the percent by weight of water in this hydrate? (b) If 15.000 grams of this hydrate is heated, what mass of water would be lost? I'm thinking that I have to find out the mass of the CaSO4 and then the mass of 2H2O, then...- hairy_grape
- Thread
- Percent Weight
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
K
What is the percent abundance of 140Ce in Cerium?
i am trying to figure out the percent abundance of two isotopes. i know how to set up the problem but i don't know how to find the weight of each isotopes. here is the problem: [SIZE="3"]Cerium (58Ce) has two commonly occurring isotopes, 140Ce and 142Ce. what is the approximate percent...- kceddis
- Thread
- Abundance Isotopes Percent
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
Y
How Do You Calculate the Percent Composition of Dopamine?
% (by mass) composition Homework Statement Dopamine, C8 H11 O2 N is a neutroransmitter. Determine the percent (by mass) composition of each of the elements in dopamite.Homework Equations The Attempt at a Solution 8C atoms= 12 (12.0) = 44.0amu 11H atoms = 11 (1.0) = 11.0 amu 2O atoms = 2...- yuuri14
- Thread
- Composition Mass Percent
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
F
Calculating Percent Uncertainty in Area of a Circle
I am asked "What is the area and it's approximate uncertainty of a circle of radius 3.8x10^4 cm?" Now finding the area is easy. In my text it says that when you aren't GIVEN an uncertainty, you can assume it is a reasonable figure in the last digit, so in this problem I am assuming the...- fattydq
- Thread
- Percent Uncertainty
- Replies: 3
- Forum: Introductory Physics Homework Help