Process Definition and 1000 Threads
-
N
Undergrad Is There Heat Transfer in a Stirling Engine's Constant Volume Process?
Does Q exist during Constant volume process in Stirling engine? It seems like Q=E=3/2nRT? but I am not quite sure.- notojosh
- Thread
- Constant Process Volume
- Replies: 1
- Forum: Thermodynamics
-
T
Undergrad Nebular Theory: Understanding Flattening Process
Hi, I try to understand the nebular theory (how the solar systems are formed) but I keep having problems with the flattening. My physical intuition "feels" the flattening process should happen, but I want a physical/mathematical qualitative explanation. What is the key physical law that... -
C
Second Order Stationary Process
Homework Statement Let X(t), -inf< t < inf, be a second order process. Show that it is a second order stationary process if and only if mu(t) sub x is independent of t and r(s,r) sub x depends only on the difference between s and t. Show that is a second order stationary process if and...- cdplayersony
- Thread
- Process Second order
- Replies: 1
- Forum: Calculus and Beyond Homework Help
-
A
Schools Grad school application process (math)
hi, so I'm just finishing up my junior year and would like to try to go to grad school for math. I got into an REU program this summer that ends around the beginning of August and plan to start studying for the GRE sometime during the summer. Right now though, I'm a little confused as to how I...- afkguy
- Thread
- Application Grad Grad school Grad school application Process School
- Replies: 4
- Forum: STEM Academic Advising
-
M
Gram-Schmidt Process: Example 3 in PDF
Homework Statement Example 3 in this pdf: http://karin.fq.uh.cu/qct/Tema_04/04.03.Los%20valores%20y%20vectores%20propios%20de%20un%20sistema/Diagonalization.pdf" Homework Equations Gram-schmidt process: v2 perp = v2 - (u1*v2)u1 The Attempt at a Solution I don't understand how they...- morsel
- Thread
- Process
- Replies: 10
- Forum: Calculus and Beyond Homework Help
-
A
Calculate the amount of heat power that is generated by the isothermal process
Homework Statement 200W of power is required for an isothermal process of a larger cyclic process. Use the first law of thermodynamics to calculate the amount of heat power that is generated by the isothermal process. Homework Equations Change in heat energy = change in internal energy +...- accidentprone
- Thread
- Heat Isothermal Power Process
- Replies: 3
- Forum: Introductory Physics Homework Help
-
A
C/C++ HELP How to process multiple files in C++
HELP! How to process multiple files in C++ I have been bashing my head against my desk in my cubical trying to figure this out. I have approximately 500 files I would like to read in, one at a time, into a C++ program, do my processing, and send the results to a new file. I can do the...- aLostProgramm
- Thread
- C++ files Multiple Process
- Replies: 4
- Forum: Programming and Computer Science
-
M
Polytropic Process: Finding Final Pressure and Volume
Homework Statement A frictionless piston cylinder contains ideal gas hydrogen , R = 4.125 KJ/Kg.K , initially at p1=100 Kpa , t1 = 765 C , V1 = 0.35 m3 . The gas undergoes a polytropic process with n = 1.22 until the final volume becomes V2=8.0 m3 Cp = 14.3338 , Cv= 10.2043 ...- Mohd17
- Thread
- Polytropic Process
- Replies: 5
- Forum: Introductory Physics Homework Help
-
S
Is delta H=0 for isothermal process?
1.>Consider a gas in a vessel with a piston on top.Let it expand to a greater volume. So, delta H=delta U+delta(PV) but delta U is 0 as it is isothermal. now,as the number of moles of gas remains constant,delta(PV) is nR(delta T) which is again 0. SO delta H is 0. Thats what my book says...- sachin123
- Thread
- Delta Isothermal Process
- Replies: 4
- Forum: Introductory Physics Homework Help
-
S
Graduate Understanding Quasi-Static Process: Work and Temperature Changes
Consider a quasi-static expansion of a gas. If you change the external force by dFext, then the system will do work on the surroundings until the internal pressure equals the external pressure, right? Now, how does the temperature of the system and the surroundings chnage in the process...- spaghetti3451
- Thread
- Process Quasi-static
- Replies: 3
- Forum: Thermodynamics
-
J
Undergrad Photosynthesis converts CO2 into sugars, can we industrialize this process?
CO2 appears to create global warming, but is there a way to get rid of the CO2? Could we somehow industrialize the photosynthesis process to get rid of large amounts of CO2? What sorts of issues prevent this solution?- JDoolin
- Thread
- Co2 Photosynthesis Process
- Replies: 5
- Forum: Other Physics Topics
-
V
Graduate How Do Stochastic Processes Apply to Real-World Events and Systems?
1. Assume that earthquakes strike a certain region at random times that are exponentially distributed with mean 1 year. Volcanic eruptions take place at random times that are exponentially distributed with mean 2 years. What is the probability that there will be two earthquakes before the next...- vampire2008
- Thread
- Process Stochastic Stochastic process
- Replies: 2
- Forum: Set Theory, Logic, Probability, Statistics
-
V
Modeling Random Processes in Natural Phenomena: Case Studies and Applications
Homework Statement 1. Assume that earthquakes strike a certain region at random times that are exponentially distributed with mean 1 year. Volcanic eruptions take place at random times that are exponentially distributed with mean 2 years. What is the probability that there will be two...- vampire2008
- Thread
- Process Stochastic Stochastic process
- Replies: 8
- Forum: Calculus and Beyond Homework Help
-
D
What is the Induction Process for Finding det(alpha*A)?
Old topic: https://www.physicsforums.com/showthread.php?t=194725 I have a question that's the same as the one in the old topic/thread I linked to above. At the last post, I am trying to figure out how to carry out the induction process. \sum_{j=1}^n (-1)^{i+j} a_{ij} det(\alpha A_{ij})...- DMOC
- Thread
- Induction Process
- Replies: 1
- Forum: Calculus and Beyond Homework Help
-
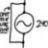
Is There a Foolproof Method for Solving BJT Circuit Problems?
Homework Statement I am just curious whether or not there is a step-by-step algorithm that will always produce the desired results when solving BJT circuits? We are learning them in my electronics course at the moment, and it seems as though the approach to the problems is never...- jegues
- Thread
- Process
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help
-
N
Graduate Spatial statistics - Point process on a network of one-dimensional lines
Hi everyone, My data consists of road accidents and spatial covariates. Originally i wanted to apply an inhomogenous Poisson process with covariates. Then I realized that the accidents cannot occur everywhere in space, but only on roads. I found an example, that is quite similar to mine. it...- Nemorad
- Thread
- Lines Network Point Process Statistics
- Replies: 7
- Forum: Set Theory, Logic, Probability, Statistics
-
B
Isothermal, adiabatic, isovolumetric process
Homework Statement Air with the mass of 10kg, with pressure 15 bar and temperature 50 C comes to a isobaric expansion to 3 times the original volume, then the air cools to the pressure of 6 bar(isovolumetricly). After that it comes to adiabatic expansion to original temperature (50 C i guess)...- Bassalisk
- Thread
- Adiabatic Isothermal Process
- Replies: 6
- Forum: Advanced Physics Homework Help
-
S
Undergrad Definition of a reversible process
I have seen a reversible process defined as one in which the system and surroundings are restored to their initial state without change elsewhere. As far as I am aware, the system and the surroundings completely occupy the universe. So, I am failing to understand what elsewhere means in this...- spaghetti3451
- Thread
- Definition Process Reversible Reversible process
- Replies: 9
- Forum: Thermodynamics
-
B
Ideal gas undergoes cycle process
Hi, An amount of ideal gas undergoes the folowing cycle process in the following graph: This process can be presented by the following PV-graph : The answer has to be the upper graph but why can't it be the lower graph ? Thank you!- b_andries
- Thread
- Cycle Gas Ideal gas Process
- Replies: 1
- Forum: Introductory Physics Homework Help
-
E
Solving Isothermal Process: Heat Supplied at Constant Pressure & Volume
Homework Statement I have a diagram of a process of a monoatomic gas. and the problem is that I know that the total amount of work = total amount of heat, so there is no internal energy change. why? because my process started at the same temp that it finished. how ever... it is asking me...- eliassiguenza
- Thread
- Isothermal Process
- Replies: 1
- Forum: Introductory Physics Homework Help
-
I
Medical Kidney's process of reabsorpting the water
how could the process of the kidney's process of reabsorpting the water be simulated through a man made experiment?- isyang94
- Thread
- Process Water
- Replies: 1
- Forum: Biology and Medical
-
A
Graduate Work done for an adiabatic process
Hi, In deriving the equation for adiabats : V^{\gamma}P = C. It is assumed that the work done during the process is W = -P \Delta V. But calculating the work done from V^{\gamma}P = C we obtain: W = \frac{C}{1-\gamma}\left[ V^{1-\gamma}_{f} - V^{1-\gamma}_{i} \right] . Also, i believe to... -
X
Undergrad Why is the change in enthelpy 0 during an isothermal process?
I am reviewing material for my thermodynamics class and one of the practice exams says that the change in enthalpy during an isothermal process is equal to zero. This doesn't make any sense to be considering.. \Delta H = \Delta U + PV and since this is an isothermal process.. \Delta U =...- Xyius
- Thread
- Change Isothermal Process
- Replies: 12
- Forum: Thermodynamics
-

Graduate Looking for the name of a process.
Suppose we have two Gaussian distributions with means A and B. These systems have probability P at time T to be located at X. These are the probability distributions of a simple one-dimensional Wiener process. M=mean P(M,T)=1/sqrt(2*pi*T) * exp(-(X-M)^2/(2*T)) So: P(A...- Rlam90
- Thread
- Process
- Replies: 1
- Forum: Quantum Physics
-
W
Reactor volume in anaerobic biodegradation process
hello, today I faced a problem with explaining why in anaerobic process the volume of UASB reactor is smaller than volume in CSTR with the same amount of final product - biogas Answer should contain characteristics like: difference in kinetics, concentration, rxn yield. Can anybody explain...- War-Saw
- Thread
- Process Reactor Volume
- Replies: 1
- Forum: Materials and Chemical Engineering
-
D
Supercooled water freezes. Is this a spontaneous process?
Supercooled water freezes. Is this a spontaneous process?? Homework Statement "one mole of supercooled water at -10degC freezes reversibly to ice at -10degC at constant P of 1500atm. The system is at chemical equilibrium. Assume that ΔH_fusion is independent of temperature. Also assume that...- Dars
- Thread
- Process Spontaneous Water
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
S
High School Making New Paper Process and Making Recycled Paper Process
What is the main different within them process ? Any good web or source can gain more knowledge about them?- soonsoon88
- Thread
- Paper Process
- Replies: 8
- Forum: Other Physics Topics
-
A
Where Can I Find Free Process and Reaction Simulation Software?
Hi, Do anyone know where to get free process simulation software, please, if you know let me know, thanks. -
F
Graduate Electrostatic energy in annihilation process
When an electron and a positron annihilate, they typically produce two gamma rays, each of energy mc^2 plus whatever kinetic energy available before annihilation. I was recently told that it is an experimental fact that the electrostatic energy between the electron and the positron does NOT...- FredMadison
- Thread
- Annihilation Electrostatic Electrostatic energy Energy Process
- Replies: 7
- Forum: Quantum Physics
-
V
How Does Constant Pressure Affect Heat Transfer in Oxygen?
Homework Statement Suppose 15.2 g of oxygen (O2) is heated at constant atmospheric pressure from 13.3°C to 148°C. (a) How many moles of oxygen are present? (Take the molar mass of oxygen to be 32.0 g/mol) (b) How much energy is transferred to the oxygen as heat? (The molecules rotate but do...- VitaX
- Thread
- Constant Constant pressure Pressure Process
- Replies: 6
- Forum: Introductory Physics Homework Help
-
F
Graduate Entropy change of an irreversible process
i can't seem to get something in classical thermodynamics entropy is a state function of a system,that means it only depends on the state of a system not time or path. if i have an irreversible process between state 1 and state 2, and back to state 1, and i want to know the entropy change of...- flasherffff
- Thread
- Change Entropy Irreversible Process
- Replies: 2
- Forum: Thermodynamics
-
0
Relative Motion - What is your thought process?
This is not a homework question. I know the answer to this question I'm just not sure how, from reading the question, I would have known what the question is actually asking. If someone could explain what their thought process is after reading the question, and how it is that you know what the...- 03125
- Thread
- Motion Process Relative Relative motion
- Replies: 2
- Forum: Introductory Physics Homework Help
-
M
Thermodynamics problem- isobaric process
Homework Statement One mole of an ideal monatomic gas is taken through the cycle ABCA shown schematically in the diagram. State A has volume 0.0208 m3 and pressure 1.22 × 10 5 Pa, and state C has volume 0.0524 m3 Process CA lies along the 305 ±1 K isotherm. The molar heat capacities for...- mrdith007
- Thread
- Process Thermodynamics
- Replies: 1
- Forum: Introductory Physics Homework Help
-
C
Thermodynamics - Adiabatic Process
Homework Statement A cylinder, A, containing a monatomic ideal gas, is fitted with a frictionless leakfree piston. The axis of the cylinder is oriented vertically, as in the figure below, so that the weight of the piston maintains the gas at a constant pressure P0. The cylinder is linked...- catdog90210
- Thread
- Adiabatic Adiabatic process Process Thermodynamics
- Replies: 1
- Forum: Introductory Physics Homework Help
-
Z
What is the Concept Behind 'Irreversible Adiabatic Process'?
What is the concept behind 'irreversible adiabatic process' ? Why is the expression for work done in this case different from that when it is reversible? -
D
Medical Opponent process theory: why do I see green after looking at white?
This morning, I was lying on my bed, looking at the bright morning light coming in through the window. The blinds (which are white) were at least half shut, so much of the light was filtered through them. I then stared up at the ceiling, and saw a nebulous patch of green. I shut my eyes, and...- DeuteriumDude
- Thread
- Green Process Theory
- Replies: 3
- Forum: Biology and Medical
-
R
Graduate Finding the relesed energy in decay process
Hi, Radioactive materials will decay to datughter products. in the mean process they emit alpha or beta particles with some energy and then decay to new products. In my problem i am just observing U(238) decay process. I need to find the energy values for each decay and also i need...- rama1001
- Thread
- Decay Energy Process
- Replies: 2
- Forum: High Energy, Nuclear, Particle Physics
-
K
Graduate Is There Any Reversible Quasi-Static Process?
Just out of curiosity, is there any quasi-static process that is completely reversible? I think the answer is an obvious no because any quasi-static process experiences a net energy loss but I'd like to know if there are any, possibly something abstract rather then mechanical.- Kevin_Axion
- Thread
- Process Quasi-static
- Replies: 2
- Forum: Thermodynamics
-
J
Undergrad The process involved in reflection?
when we have light reflecting off a material, what's going on there? is it something analogous to a gas particle rebounding off a container wall due to Coulomb repulsion? If so, what is it that exerts a force to reverse the photon's momentum perpendicular to the surface it's reflecting off...- jeebs
- Thread
- Process Reflection
- Replies: 10
- Forum: Quantum Physics
-
V
Undergrad Adiabatic Process and the speed of sound
Hi all, Sorry if this post is a bit wordy but I've been going round in circles and I thought I'd see if anyone on here can help, it's also my first post so be nice... I've been trying to understand how Newton miscalculated the speed of sound. I know that he thought that the propagation of...- veryownjustin
- Thread
- Adiabatic Adiabatic process Process Sound Speed Speed of sound
- Replies: 4
- Forum: Mechanics
-
R
How to calculate the heat added during an isothermal process
Homework Statement The titleHomework Equations ?The Attempt at a Solution The only ways I know of to calculate heat are dQ = Cp.dT and dQ = Cv.dT for isochoric and isobaric process how would you go about doing it for an isothermal process Also if a system isn't a cycle but it ends up in the...- renlok
- Thread
- Heat Isothermal Process
- Replies: 1
- Forum: Advanced Physics Homework Help
-
S
Thermodynamics 1st Law: reversible process for batteries?
How could you ensure that an electric battery produced an electric current reversibly? How could you achieve maximum work from an electric battery? I really have no idea how to solve this. Thanks for your help! (Also sorry I first posted this in advanced physics...i don't know how to...- sparkle123
- Thread
- Batteries Law Process Reversible Reversible process Thermodynamics
- Replies: 1
- Forum: Introductory Physics Homework Help
-
J
Graduate Feynman rules and decay process
Hi I need help to understand Feynman rules for decay for exams. In past paper there is the following question as whether the following are allowed. cc decays to tau++tau-. From What I can see this is possible via the strong force is this correct? The next is cc decays to cu and cu. From...- jc09
- Thread
- Decay Feynman Feynman rules Process Rules
- Replies: 3
- Forum: High Energy, Nuclear, Particle Physics
-
U
Adiabatic process thermodynamics help
Homework Statement An ideal diatomic gas at 75 K is adiabatically compressed to 40% its original volume. What is its final temperature? Homework Equations delta U = Q - W W = the integral of PdV PVgamma = constant U = 5/2nKT = 5/2nRT (diatomic, ideal gas) The Attempt at a Solution...- uchicago2012
- Thread
- Adiabatic Adiabatic process Process Thermodynamics
- Replies: 2
- Forum: Introductory Physics Homework Help
-
J
Graduate What is a first order process in particle physics
I was given the following question Which of the reactions on the right are allowed by first-order processes? For those which are not allowed, state one conservation law which is violated. What is a first order process in particle physics? i.e what does first order refer to? What would be...- j-lee00
- Thread
- First order Particle Particle physics Physics Process
- Replies: 7
- Forum: High Energy, Nuclear, Particle Physics
-
L
What is a process corner and why does it impact semiconductor speed and quality?
What exactly is a process corner. Is it actually the corner on the silicon wafer? Why does speed vary in process corners? Is the doping not uniform?- likephysics
- Thread
- Process Semiconductor
- Replies: 5
- Forum: Electrical Engineering
-
P
Specific heat in izobaric process
Homework Statement how can I calculate specific heat at constant pressure from mass specific heat and mole mass?- player1_1_1
- Thread
- Heat Process Specific Specific heat
- Replies: 3
- Forum: Introductory Physics Homework Help
-
D
What is the final pressure of an adiabatically compressed gas in a container?
Homework Statement a container with air (M = 29 g/mol, treated as an ideal gas). In the container, we have 2.0 L of the gas with p1 = 1.0 atm at T1 = – 50C. The gas is compressed adiabatically to a final volume of 0.5 L. What is its final pressure? Homework Equations I am really not...- de3tz
- Thread
- Adiabatic Adiabatic process Process
- Replies: 1
- Forum: Introductory Physics Homework Help
-
M
Graduate Isobaric Process finding G using (deltaG-deltaGo)
Can anyone help me with understanding what is going on at page 11 of these notes? http://www.chem.utoronto.ca/coursenotes/CHM223/Section%205B%20Fall%202010.pdf Do you get why there is 2 delta Gs? I am really confused with what he was trying to do there? ΔG(37oC) – ΔG(25oC) ≈ – (T – 298)...- mikece
- Thread
- Process
- Replies: 1
- Forum: Thermodynamics
-
T
Thermodynamics problem - adiabatic process
Homework Statement One cylinder in the diesel engine of a truck has an initial volume of 600 cm^3. Air is admitted to the cylinder at 35 C and a pressure of 1.0 atm. The piston rod then does 500 J of work to rapidly compress the air. What is the final temperature and volume? I found the final...- Twoism
- Thread
- Adiabatic Adiabatic process Process Thermodynamics
- Replies: 5
- Forum: Introductory Physics Homework Help