- #1
curiosity colour
- 21
- 0
Please post this type of questions in HW section using the template and showing your work.
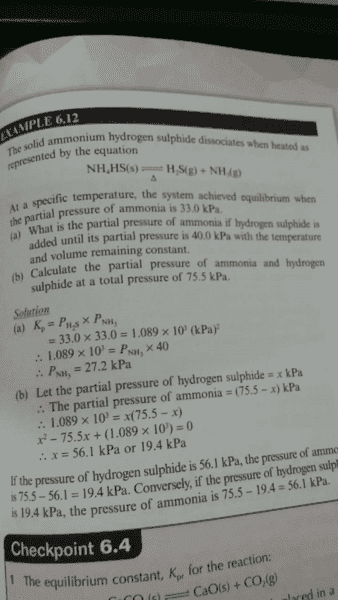
I have a question about the solution (a) in this example, how does it know PH2S is the same as ammonia? is there something I've miss?