- #1
mcfaker
- 43
- 0
Hi,
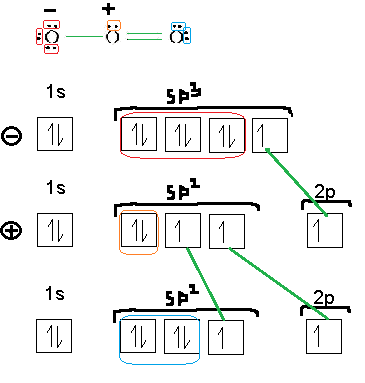
Could someone tell me if the following orbital diagram is correct for ozone? I added the bonds and lone pairs in color.
 Thanks in advance.
Thanks in advance.
Could someone tell me if the following orbital diagram is correct for ozone? I added the bonds and lone pairs in color.