You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Hydrogen: Definition and 1000 Discussions
Hydrogen is the chemical element with the symbol H and atomic number 1. With a standard atomic weight of 1.008, hydrogen is the lightest element in the periodic table. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all baryonic mass. Non-remnant stars are mainly composed of hydrogen in the plasma state. The most common isotope of hydrogen, termed protium (name rarely used, symbol 1H), has one proton and no neutrons.
The universal emergence of atomic hydrogen first occurred during the recombination epoch (Big Bang). At standard temperature and pressure, hydrogen is a colorless, odorless, tasteless, non-toxic, nonmetallic, highly combustible diatomic gas with the molecular formula H2. Since hydrogen readily forms covalent compounds with most nonmetallic elements, most of the hydrogen on Earth exists in molecular forms such as water or organic compounds. Hydrogen plays a particularly important role in acid–base reactions because most acid-base reactions involve the exchange of protons between soluble molecules. In ionic compounds, hydrogen can take the form of a negative charge (i.e., anion) when it is known as a hydride, or as a positively charged (i.e., cation) species denoted by the symbol H+. The hydrogen cation is written as though composed of a bare proton, but in reality, hydrogen cations in ionic compounds are always more complex. As the only neutral atom for which the Schrödinger equation can be solved analytically, study of the energetics and bonding of the hydrogen atom has played a key role in the development of quantum mechanics.
Hydrogen gas was first artificially produced in the early 16th century by the reaction of acids on metals. In 1766–81, Henry Cavendish was the first to recognize that hydrogen gas was a discrete substance, and that it produces water when burned, the property for which it was later named: in Greek, hydrogen means "water-former".
Industrial production is mainly from steam reforming natural gas, and less often from more energy-intensive methods such as the electrolysis of water. Most hydrogen is used near the site of its production, the two largest uses being fossil fuel processing (e.g., hydrocracking) and ammonia production, mostly for the fertilizer market. Hydrogen is problematic in metallurgy because it can embrittle many metals, complicating the design of pipelines and storage tanks.
View More On Wikipedia.org
The universal emergence of atomic hydrogen first occurred during the recombination epoch (Big Bang). At standard temperature and pressure, hydrogen is a colorless, odorless, tasteless, non-toxic, nonmetallic, highly combustible diatomic gas with the molecular formula H2. Since hydrogen readily forms covalent compounds with most nonmetallic elements, most of the hydrogen on Earth exists in molecular forms such as water or organic compounds. Hydrogen plays a particularly important role in acid–base reactions because most acid-base reactions involve the exchange of protons between soluble molecules. In ionic compounds, hydrogen can take the form of a negative charge (i.e., anion) when it is known as a hydride, or as a positively charged (i.e., cation) species denoted by the symbol H+. The hydrogen cation is written as though composed of a bare proton, but in reality, hydrogen cations in ionic compounds are always more complex. As the only neutral atom for which the Schrödinger equation can be solved analytically, study of the energetics and bonding of the hydrogen atom has played a key role in the development of quantum mechanics.
Hydrogen gas was first artificially produced in the early 16th century by the reaction of acids on metals. In 1766–81, Henry Cavendish was the first to recognize that hydrogen gas was a discrete substance, and that it produces water when burned, the property for which it was later named: in Greek, hydrogen means "water-former".
Industrial production is mainly from steam reforming natural gas, and less often from more energy-intensive methods such as the electrolysis of water. Most hydrogen is used near the site of its production, the two largest uses being fossil fuel processing (e.g., hydrocracking) and ammonia production, mostly for the fertilizer market. Hydrogen is problematic in metallurgy because it can embrittle many metals, complicating the design of pipelines and storage tanks.
View More On Wikipedia.org
-
H
Electron wavefunction in hydrogen
Hi. I would love if someone could check my solution since me and the answer sheet I found online don't agree. The probability is given by the triple integral \begin{align*} \int_0^{r_b} \int_0^{2\pi} \int_0^\pi |\psi (r)|^2 r^2 \sin{\theta} \,d\theta \,d\phi \,dr &= \frac{1}{\pi...- hicetnunc
- Thread
-
- Tags
- Electron Hydrogen Wavefunction
- Replies: 7
- Forum: Advanced Physics Homework Help
-

I More info on HT/HD shielding constants?
I was wondering how they measured or calculated these differences? I don't know what they refer to, but assume theyre scattering Hydrogen with Tritium or Deuterium to measure the difference of something. https://physics.nist.gov/cgi-bin/cuu/Results?search_for=shielding+difference -

I Rotation curve with neutral hydrogen and dark matter
Flat rotation curve in galaxies is determined by observing neutral hydrogen which is co-distributed with dark matter. What is the rotation curve profile of neutral hydrogen in galaxies where there is less dark matter?- Ranku
- Thread
- Replies: 7
- Forum: Astronomy and Astrophysics
-
O
I General solution of the hydrogen atom Schrödinger equation
Hello everyone! I have two questions which had bothered me for quite some time. I am sorry if they are rather trivial. The first is about the general solution of the hydrogen atom schrödinger-equation: We learned in our quantum mechanics class that the general solution of every quantum system...- Oliver321
- Thread
- Replies: 9
- Forum: Quantum Physics
-
P
B Can a hydrogen atom become a neutron?
I read this from Nasa's website: "Within the first second after the Big Bang, the temperature had fallen considerably, but was still very hot - about 100 billion Kelvin (1011 K). At this temperature, protons, electrons and neutrons had formed, but they moved with too much energy to form atoms...- ProjectFringe
- Thread
-
- Tags
- Atom Hydrogen Hydrogen atom Neutron
- Replies: 5
- Forum: High Energy, Nuclear, Particle Physics
-
H
Calculating Pressure & Mass for Hydrogen Tank Filling
As I stated in the summary I'd like to calculate the required pressure and mass of a tank of hydrogen that would allow me to fill a second tank with 10kg of H2 at 700bar. I am trying to get an idea of the feasibility of doing this, putting aside, for now, how the primary tank would itself be... -
J
Hydrogen in the Aviation Industry -- Survey
Hello Everyone- I'm completing my Master's Degree in Commercial Aviation. I'm conducting a capstone research project about the potential of an industry shift to hydrogen, and have created a quick survey. If you have additional insight in addition to the survey, please respond in the comments... -

Verify the average value of (1/r) for a 1s electron in the Hydrogen atom
For spherical coordinate, ##dV = r^{2} \sin {\theta} dr d\theta d\phi## Therefore, $$<\frac{1}{r}> = \frac{1}{(\pi)(a_0)^{3}} \int_{0}^{\infty} {r e^{\frac{-2r}{a_0}} dr \int_0^{\pi} \sin {\theta}} d\theta \int_0^{2\pi} d\phi$$ From partial integral, I've found: $$\int_{0}^{\infty} r...- agnimusayoti
- Thread
- Replies: 6
- Forum: Advanced Physics Homework Help
-
L
B Nuclear fusion question -- Calculations for Hydrogen fusing into Helium
I read in 2 books that 4 atoms of Hydrogen fuse and give 1 atom of Helium and 2 electrons, and these 2 electrons convert to light. And that the mass of the Helium is less than the mass of the 4 atoms of Hydrogen, thus that the mass lost converted to light too. But I sum up the masses of...- luckis11
- Thread
- Replies: 20
- Forum: High Energy, Nuclear, Particle Physics
-

I Solving Schrödinger's equation for a hydrogen atom with Euler's method
Hi, first-time poster here I'm a student at HS-level in DK, who has decided to write my annual large scale assignment on Schrödinger's equation. My teacher has only given us a brief introduction to the equation and has tasked us to solve it numerically with Euler's method for the hydrogen atom...- Tuca
- Thread
- Replies: 2
- Forum: Quantum Physics
-

Spherically symmetric states in the hydrogen atom
The equation $$\frac{\hbar^2}{2m}\frac{d^2u}{dr^2}-\frac{Ze^2}{r}u=Eu$$ gives the schrodinger equation for the spherically symmetric functions ##u=r\psi## for a hydrogen-like atom. In this equation, substitute an assumed solution of the form ##u(r)=(Ar+Br^2)e^{-br}## and hence find the values...- docnet
- Thread
- Replies: 2
- Forum: Advanced Physics Homework Help
-
E
A Feynman solution for the radial wave function of the hydrogen atom
Reading the classical Feynman lectures, I encounter the formula(19.53) that gives the radial component of the wave function: $$ F_{n,l}(\rho)=\frac{e^{-\alpha\rho}}{\rho}\sum_{k=l+1}^n a_k \rho^k $$ that, for ##n=l+1## becomes $$ F_{n,l}=\frac{e^{-\rho/n}}{\rho}a_n\rho^n $$ To find ##a_n## I...- emilionovati
- Thread
- Replies: 4
- Forum: Quantum Physics
-

I Z2 symmetry in the hydrogen molecule when mapping to qubits
Hello! When using a Jordan-Wigner-mapping or parity-mapping to map the hydrogen molecule \mathrm{H}_2 with two electrons and 4 spin-orbitals to 4 qubits, it is possible to reduce the number of qubits down to two [1,2,3]. The reason is apparently that the molecule has a discrete... -
L
I Hydrogen atom: Energies and eigenstates
When we say energy levels of the hydrogen atom. Are that energies of the atom or of an electron in the atom? Also corresponding states? http://hyperphysics.phy-astr.gsu.edu/hbase/quantum/hydwf.html Why energies are negative? E_n \propto \frac{-1}{n^2}- LagrangeEuler
- Thread
- Replies: 17
- Forum: Quantum Physics
-
T
1420 MHz--- the emission frequency of cold hydrogen gas
I recently finished reading Paul Davies book The Eerie Silence, which is a book about the SETI (Search for ExtraTerrestrial Intelligence) project. In The Eerie Silence, Davies says that scientists using radio telescopes to search for radio messages from space aliens set their radio telescopes... -

I Why does a hydrogen gas tube produce a hydrogen atomic spectrum?
To measure the atomic hydrogen spectrum people often uses hydrogen gas tubes as light source. Since the gas in the tube is the molecule ##H_2## , why we obtain the spectrum of atomic hydrogen? My guess is that because the voltage is so high, so that the molecules are totally dissociated. If...- amilton
- Thread
- Replies: 9
- Forum: Atomic and Condensed Matter
-

Relative volume of combusted hydrogen
Hi, If I had a volume of Brown's gas at 20°C / 1atm, what would the expected volume [of the resultant steam] be immediately after it was ignited? Thanks! Bob -
P
Chemistry Why is HCl called Hydrogen Chloride (by IUPAC naming)?
Hydrogen Monochloride- pkc111
- Thread
- Replies: 9
- Forum: Biology and Chemistry Homework Help
-

An experiment for the determination of hydrogen ionization energy
hi guys i saw this experiment in an old book that uses the gas vacuum tube "thyratron" for determining the hydrogen ionization energy , the idea i guess is straight forward : we set the filament current to a specific value then the electrons starts to emit from the cathode traveling its way to...- patric44
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
B
Hydrogen Fusion Engine: Steel Sphere & Sulfuric Acid
Have a thick steel hollow sphere with a inside radius of 20 cm. Then fill it up with sulfuric acid and add water. Then remove the oxygen ions. then give the sphere a negative charge and all the hydrogen ions move to the surface of the inside sphere. then charge sphere positive and the hydrogen...- Bruce Haawkins
- Thread
- Replies: 5
- Forum: Nuclear Engineering
-
Z
What is the Saha equation and how does it apply to hydrogen gas?
I have no clue. I am stuck. I would appreciate If someone could help me out. I just know I need to use the Saha equation.- zenodv
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-

I Exploring Dynamics of Electron in Hydrogen Atom
Hello,everyone. Can dynamical system be used to describe the behavior of the electron in Hydrogen atom?- thaiqi
- Thread
- Replies: 7
- Forum: Quantum Physics
-

I Q re a photon ionizing a hydrogen atom
This question is a followup to another thread. https://www.physicsforums.com/threads/qs-re-the-behavior-of-atoms-after-decoupling-completed.994581/ I would like to explore the issue raised by @kimbyd. . . . after reionization the temperature of the intergalactic medium is dominated by...- Buzz Bloom
- Thread
-
- Tags
- Atom Hydrogen Hydrogen atom Photon
- Replies: 1
- Forum: Quantum Physics
-

A strange wave function of the Hydrogen atom
I am trying to solve the following exercise. In a H atom the electron is in the state described by the wave function in spherical coordinates: \psi (r, \theta, \phi) = e^{i \phi}e^{-(r/a)^2(1- \mu\ cos^2\ \theta)} With a and \mu positive real parameters. Tell what are the possible values...- omegax241
- Thread
- Replies: 5
- Forum: Advanced Physics Homework Help
-

I What is the density of hydrogen atoms in the Universe?
UNITS m is meters kg is kiliograms K is degrees Kelvin s is seconds J is joules u is daltons = 1.66053906660(50)×10−27 kg https://en.wikipedia.org/wiki/Dalton_(unit) 1 pc = 3.085678 x 1016 m CONSTANTS MH = mass of hydrogen atom = 1.007825 u = 1.673532784796145 ×10−27 kg... -
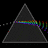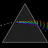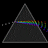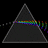
Hydrogen Emission Spectrum and Electron Energy Levels
1. The 4th line from the left, being the aqua blue line, corresponds to a wavelength of 486 nm, as blue light has a wavelength in the range 450-495 nm. 2. This is where I am having the most difficulty, I have tried to answer the question comprehensively but I am not satisfied with my answer. In...- AN630078
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-

Elastic Collision of Hydrogen and Carbon Atoms
1.p=mv Before the collision: p hydrogen = 1.7x10^-27 * 500 =8.5*10^-25 kg ms^-1 p carbon = 2.0x10^-26 * 0 = 0 kg ms^-1 p total before = 8.5*10^-25 kg ms^-1 The sum of momentum prior to the collision is equal to the total momentum after a collision, momentum is constant, therefore; p before = p...- AN630078
- Thread
- Replies: 9
- Forum: Introductory Physics Homework Help
-

Doppler effect and hydrogen alpha distributions
https://www.asi.edu.au/wp-content/uploads/2015/03/PhysicsASOE2014solutions.pdf q 14b) i) Assuming that the planet is rotating at a constant rate, shouldn't the distribution be even across all wavelengths, or do I have something very wrong with my model. I take the graph as the summation of...- aspodkfpo
- Thread
- Replies: 3
- Forum: Introductory Physics Homework Help
-

Reaction of Potassium Permanganate and Hydrogen Peroxide solution
With the peroxide solution in excess, we added 10ml vinegar and 10ml of different concentrations of permanganate and timed the reaction. The basics are that the more concentrated the permanganate , the faster the reaction. I would just like to know what the theoretical relationship is between...- neilparker62
- Thread
- Replies: 8
- Forum: Chemistry
-

I Effects of hydrogen anions in the solar atmosphere
What is the population density of ##H^-## hydrogen anions in the Sun's atmosphere, how stable are they, what limits their numbers, and since they are the dominant source of opacity in radiative transfer in stellar atmospheres, what do they effectively "hide"?- sciFax
- Thread
-
- Tags
- Atmosphere Effects Hydrogen Solar
- Replies: 4
- Forum: Astronomy and Astrophysics
-

B Hydrogen anions - how do they form?
Having read a few Wiki articles on and around the subject, I am now somewhat aware of the means by which these ##H^-## anions acquire the extra electrons "donated" by e.g. ionized alkali atoms in stellar atmospheres, and the means by which they provide opacity. What I don't understand is how it...- sciFax
- Thread
- Replies: 21
- Forum: Atomic and Condensed Matter
-

Help with 3-D interactive QM visualisation of a hydrogen atom
First of all, I got to decide what I'm going to use to make the simulation. I know Fortran, Matlab etc but I'm pretty sure these won't help me much. I learned some C++ a couple years ago but my knowledge is rusty, however I think I'm going to use that combined with Unreal Engine, since it makes...- AndreasC
- Thread
- Replies: 5
- Forum: Computing and Technology
-
G
B The Coronal Heating Problem - The Hydrogen Fusion Core disappears?
In previous manifestations explaining the Coronal Heating Problem, scientists pointed out the discontinuity between a very hot Solar interior, the 'cool' Photosphere and a Hot Corona. According to the newly modified entry in Wikipedia, they now explain the discontinuity only between a cool... -
A
B Area of a Circle in an Electron's Hydrogen Atom
My textbook says "A is the area of the circle enclosed by the current" (produced by an electron in a hydrogen atom), A = ##\pi r^2 \sin(\theta)^2##. I don't understand where the ##\sin(\theta)^2## comes from.- Anthill
- Thread
- Replies: 1
- Forum: General Math
-
K
TISE solution for a hydrogen atom
I am unable to complete the first part of the question. After I plug in the function for psi into the differential equation I am stuck: $$\frac {d \psi (r)}{dr} = -\frac 1 a_0 \psi (r), \frac d{dr} \biggl(r^2 \frac {d\psi (r)}{dr} \biggr) = -\frac 1 {a_0}\frac d {dr} \bigl[r^2 \psi(r) \bigr] =...- Kynsuo
- Thread
- Replies: 22
- Forum: Introductory Physics Homework Help
-

Quantum Mechanics Hydrogen Atom Expectation Value Problem
I can not solve this problem: However, I have a similar problem with proper solution: Can you please guide me to solve my question? I am not being able to relate Y R (from first question) and U (from second question), and solve the question at the top above...- cemtu
- Thread
- Replies: 24
- Forum: Advanced Physics Homework Help
-

Quantum Mechanics hydrogen atom eigenfunction problem
This is a general property of eigenvectors of Hermitian operators. State functions are a particular class of vector, and it is easiest to work in the general formalism (I am hoping to show how ket notation makes qm easier, not just do standard bookwork at this level). Suppose O is a Hermitian...- cemtu
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
Y
Is it possible to ionize hydrogen peroxide at home?
Hi, I wonder maybe anyone can help me. I’m trying to ionize hydrogen peroxide mist with the help of a high voltage electrical filed / corona arc. And I wonder if there is a way to know if I ionize the hydrogen peroxide mist at all? And if so, how much do I ionize it (compering to deferent...- yair252525
- Thread
- Replies: 11
- Forum: Chemistry
-
B
Is hydrogen peroxide effective against the corona virus?
If oxygen is effective against bacteria virus and cancer why isn't it used against coved-19? DMSO is known to bond with certain substances and could carry HP into the blood stream and kill off COVID-19.- bill hart
- Thread
- Replies: 1
- Forum: Biology and Medical
-
G
I Perturbation lines for the 1s hydrogen electron
Does anyone know theory about how the perturbation lines are for 1s hydrogen electron? By perturbation I mean the perturbation that is caused by moving an electron so that the E-field lines it emits becomes dragged. by perturbation I mean for example dragging a charge as described below Above...- georg gill
- Thread
- Replies: 10
- Forum: Quantum Physics
-
B
Radius of the electron orbit in a Hydrogen atom
I am really stuck on what to do here in this question I have arrived at forming an equation to work out the radius of electron orbit from doing the following However I do not know what to do next as I don't know what the value of n (quantum number) must be? :oldconfused: Any help would be...- Bolter
- Thread
- Replies: 15
- Forum: Introductory Physics Homework Help
-

Perturbation treatment of hydrogen molecular ion
hi guys i am a the third year undergrad student and in this 2nd semester in my collage we should start taking quantum mechanics along with molecular physics , our molecular physics professor choose a book that we are going to take which is " molecular physics by wolfgang Demtroder " when i...- patric44
- Thread
- Replies: 15
- Forum: Advanced Physics Homework Help
-
P
Hydrogen bonding preference in acids
I can't figure out why hydrogen is more likely to bond to either the nitrogen or to the sulphur atom. I can't locate any information in any textbooks or online as to which molecule is more prevalent/common. This is not a homework question. I just came across one of these molecules and wondered...- PhysicsCanuck
- Thread
- Replies: 2
- Forum: Chemistry
-
L
I Is the potential energy always negative in the ground state of a hydrogen atom?
Why energy of the electron in ground state of hydrogen atom is negative ##E_1=-13,6 \rm{eV}##? I am confused because energy is sum of kinetic and potential energy. Kinetic energy is always positive. How do you know that potential energy is negative in this problem?- LagrangeEuler
- Thread
- Replies: 12
- Forum: Quantum Physics
-
J
How can hydrogen gas can be made to emit photons of different energies
This above is the diagram I'm not too sure about the solution to this problem as to why I came here. Is it something to do with photons having different frequencies i.e emitting different amounts of energy based on its frequency -

I What is hydrogen boron fusion
Hello All: Read an article about new trend in fusion science about hydrogen-boron 11 fusion Called HB-11 fusion and a start up are doing a research on it using pulsed laser What I don't understand how it is considered a possible new method for energy production when we know hydrogen and...- hagopbul
- Thread
- Replies: 5
- Forum: High Energy, Nuclear, Particle Physics
-

Why is the Hydrogen Spectrum Ambiguous?
I was asked in a test this question: Electron in hydrogen falls from level 4 , how many lines we will see on the ejection spectrum? I hope I translated it well. I see a lot of question about those lines but can’t find information about it. Can anyone explain it to me? -

Is reducing energy losses critical for the success of hydrogen in the gas grid?
A trial has commenced at Keele University in the UK where 20% Hydrogen is injected into the domestic gas supply. In this way it can be stored and then utilised by existing domestic burners. Can this be a quick solution to reducing domestic CO2 emissions? Or is it better to use battery storage...- tech99
- Thread
- Replies: 51
- Forum: Earth Sciences