Energy levels Definition and 267 Threads
-

Undergrad Physical Meaning of Atomic Oscillations
A physics question for those more atomically inclined than myself. Atomic clocks are said to measure the frequency of oscillations. By this definition of atomic oscillation, is anything physically vibrating, or does it just mean switching between the two energy levels without excess explicit...- Sciencemaster
- Thread
- Atomic clock Atomic orbitals Energy levels Oscillations Vibrations
- Replies: 11
- Forum: Quantum Physics
-

Do you graph the energy levels of a ##1s^1## atom the same as a ##1s^2## atom?
From the first equation, there are 5 constants, e, ##\pi##, ##\varepsilon_0##, ##n^2##, and 32. The only difference is m, where helium has around four times the mass of hydrogen. What I don't get is if there is a difference between the energy levels of the hydrogen and the ionized hellium? Also...- Danielk010
- Thread
- Atom Energy Energy levels
- Replies: 12
- Forum: Introductory Physics Homework Help
-

Undergrad Electromagnetic radiation, photons, quantized energy levels
Hello! Im a freshman in college, taking pretty basic chem classes and Ive found myself in a deep dive regarding quantum physics. Im sure this is pretty simple and easy compared to everyone else on here but I feel like I keep getting oversimplified answers that just leave me with more...- asf33
- Thread
- Electromagnetic radiation Energy levels Quantum physics
- Replies: 1
- Forum: Quantum Physics
-
S
Is the Photoelectric Effect Proof That Light Is a Particle?
We know that electrons bound to an atom can only absorb light with certain energies that match the energy difference between two energy levels or otherwise this implies electrons can exist in between energy levels. Then electrons will spiral into the nucleus due to the attractive forces between...- sss1
- Thread
- Energy levels Photoelectric effect
- Replies: 35
- Forum: Introductory Physics Homework Help
-
P
A system of independent particles (energy levels)
Hi guys, Can you give me some feedback on whether my calculation is correct? I applied the formula below (Boltzmann Distribution) but I didn‘t know what to use for the variable z. I don‘t even know if I used the correct equation. Can you help me further? The task is: Consider a system of...- physicisttobe
- Thread
- Energy levels Independent Levels Particles Statisical mechanics System Thermodynamics
- Replies: 1
- Forum: Advanced Physics Homework Help
-

High School What are the energy eigenvalues of a harmonic oscillator?
Is it a total energy of a vibrating molecule? So is it a sum of potential and kinetic energy? Or it is only a total energy of a vibrational motion of the molecule? Or is it only a potencial energy, when it is related to a dissociation curve? I am confused.- Lotto
- Thread
- Energy Energy levels Mean
- Replies: 2
- Forum: Quantum Physics
-

High School Atomic Energy Levels: Simplified Explanations for the Curious
I find this very interesting. But it is above my head. Is there a simpler explanation/volume perhaps that I could get, consult? https://www.sciencedirect.com/topics/earth-and-planetary-sciences/atomic-energy-levels- abrogard
- Thread
- Atomic Curious Energy Energy levels Levels
- Replies: 2
- Forum: Atomic and Condensed Matter
-
B
Graduate Energy levels shifts in a time-varying electric field
Hello! I have 2 levels of the same parity with energies ##E_1 < E_2##, and another level of opposite parity a distance ##E## from the ##E_2##. I also have that ##E_2 - E_1 << E##. I have a laser on resonance (I am trying to scan along the resonance and find it) with the transition from ##E_2##...- BillKet
- Thread
- Electric Electric field Energy Energy levels Field Levels
- Replies: 1
- Forum: Atomic and Condensed Matter
-
M
Undergrad Energy levels of atoms and spectroscopic analysis
Hello ! My question is how the energy levels and sublevels that atoms are considered to have were obtained, and if these energy levels and sublevels were obtained as a result of the different spectroscopic analyzes of energy emission and absorption of atoms, or as it was concluded that atoms...- MartinG
- Thread
- Analysis Atoms Energy Energy levels Levels
- Replies: 13
- Forum: Atomic and Condensed Matter
-
B
Graduate Time dependent perturbation theory applied to energy levels
Hello! I am reading this paper and in deriving equations 6/7 and 11/12 they claim to use second oder time dependent perturbation theory (TDPT) in order to get the correction to the energy levels. Can someone point me towards some reading about that? In the QM textbooks I used, for TDPT they just...- BillKet
- Thread
- Applied Energy Energy levels Levels Perturbation Perturbation theory Theory Time Time dependent
- Replies: 5
- Forum: Atomic and Condensed Matter
-
B
Graduate Trying to reproduce the energy levels of a molecule from a paper
Hello! This is quite a specific question, so if anyone knows the details I would really appreciate your help (@Twigg ?). I am trying to reproduce figure 1 from this paper (it's for the PV experiment performed on BaF). While I am getting quite close to it, the levels don't fully match (I am...- BillKet
- Thread
- Energy Energy levels Levels Molecule Paper
- Replies: 5
- Forum: Atomic and Condensed Matter
-

Molecular covalent bonds across energy levels
I am sure this is an elementary question; I'm just trying to clarify some points that were poorly explained to me years ago in secondary school. I know that a full answer would involve solving Schrödinger's equation etc., but keeping this on the level of valence electrons,...) I was confused by... -
M
Partition Function for system with 3 energy levels
I determined the partition function of the particle A, B and C. C should be the same as B. I then considered the situation, where all particles are in the system at the same time, and drew a diagram of all possible arrangements: The grey boxes are the different partitions, given that we...- MigMRF
- Thread
- Energy Energy levels Function Levels Partition Partition function System Thermodynamics
- Replies: 1
- Forum: Advanced Physics Homework Help
-

Undergrad Continuous Spectrum and Energy levels of Electrons (Energy Bands)
My book says that emission spectra are produced when an electron in excited state jump from excited to lower energy states. It also states that solids and liquids produce continuous spectra and it depends upon temperature only (is this black body radiation?). I know, Electrons around a nucleus...- ktmsud
- Thread
- Atomic spectra Black body radiation Continuous Electrons Emission spectrum Energy Energy levels Levels Spectrum
- Replies: 7
- Forum: Quantum Physics
-
K
Undergrad Gauge in the Aharonov Bohm effect
In p.385 of Griffiths QM the vector potential ##\textbf{A} = \frac{\Phi}{2\pi r}\hat{\phi}## is chosen for the region outside a long solenoid. However, couldn't we also have chosen a vector potential that is a multiple of this, namely ##\textbf{A} = \alpha \frac{\Phi}{2\pi r} \hat{\phi}## where...- KDPhysics
- Thread
- Aharonov-bohm Electromagnetism Energy levels Gauge Quantum
- Replies: 4
- Forum: Quantum Physics
-
K
Undergrad How to measure a 10 Hz energy splitting of two energy levels
Hello! If I have 2 energy levels split by something of the order of 10 Hz (they can be connected by an electric dipole moment i.e. ##\Delta J = 0## and they have different parities), what would be the best way to measure this difference (even 10% error would be good, but the lower the error the...- kelly0303
- Thread
- Energy Energy levels Levels Measure Splitting
- Replies: 29
- Forum: Atomic and Condensed Matter
-

Undergrad Can Having Electrons in Energy Levels Indicate Ionisation of Atoms?
Homework Statement:: Ionised atom, free electron, conduction band, donor energy level and acceptor energy level Relevant Equations:: None I have some confusion about the concept of some electronic bands and energy levels. Beyond valance band, in a solid crystal lattice, For an atom, can...- cemtu
- Thread
- Atoms Conduction band Electrons Energy Energy levels Levels Semiconductor physics
- Replies: 2
- Forum: Atomic and Condensed Matter
-
R
Undergrad Photon Emitted without Changing Energy Levels
In Example 41.5, they are implying that, for a hydrogen atom, if the orbital quantum number ##l## goes down the electron will lose energy. However, they said nothing about the principal quantum number ##n## going down, so there should be no loss in energy. As far as this book has presented, the...- rtareen
- Thread
- Energy Energy levels Levels Photon Zeeman effect
- Replies: 8
- Forum: Quantum Physics
-

High School Does the energy of an electron vary in the sublevels?
So I read that Bohr's atom has discrete energy levels that an Electron can orbit at and that each level has n amount of sublevels (if n = 2 then there are 2 sublevels). Does the sublevel that the Electron is in have to do with it's mass? Does an electron in energy level l and sublevel d have...- AdvaitDhingra
- Thread
- Electron Electron orbitals Energy Energy levels
- Replies: 10
- Forum: Quantum Physics
-
D
Calculate the Energy Levels of an Electron in a Finite Potential Well
Thank you for reading :bow: Section 1 To find the energy states of the particle, we define the wave function over three discrete domains defined by the sets ##\left\{x<-L\right\}##, ##\left\{-L<x<L\right\}##, and ##\left\{L<x\right\}##. The time independent Schrodinder equation is...- docnet
- Thread
- Electron Energy Energy levels Finite Levels Potential Potential well
- Replies: 4
- Forum: Advanced Physics Homework Help
-

Other Maintaining psychological energy levels
Many of you would have heard about physical energy levels. Like maintaining the physical energy throughout the day so that you do not get tired. People recommend exercising, meditating etc etc. But I found out that my mental energy drains at a very rapid rate. I wake up with full energy but...- sahilmm15
- Thread
- Energy Energy levels Levels
- Replies: 17
- Forum: STEM Academic Advising
-
T
Undergrad Quantum numbers for energy levels
Hello Can some one explain how you work out the combinations of quantum numbers for infinite wells in higher dimensions? For example if i have an energy level $$E_4$$ In a 2D well, then for quantum numbers does this mean the combinations allowed must be: $$4^2 + 1^2$$ $$1^2 + 4^2$$ So then...- TheCelt
- Thread
- Energy Energy levels Levels Numbers Quantum Quantum numbers
- Replies: 12
- Forum: Quantum Physics
-

Undergrad Is Probability in Quantum States Proportional to Energy Levels?
Given a particle in a 1D box with a finite number of states ##m##, is the probability a particle is in a certain state ##n## equal to the energy of that state divided by the sum of energies of all states? In other words, given $$ E_n = \dfrac{n^{2}h^{2}}{8mL^{2}}$$ is $$P_n=...- bob012345
- Thread
- Energy Energy levels Levels Probability
- Replies: 32
- Forum: Quantum Physics
-
S
High School Question about energy needed by electron to change energy levels
Electron can move from lower energy level to higher energy level when it absorbs energy equal to the difference between the energy levels based on equation: ##hf = \Delta E## If the incident photon has lower energy compared to ##\Delta E##, then electron won't move to higher energy level. But...- songoku
- Thread
- Change Electron Energy Energy levels Levels
- Replies: 6
- Forum: Quantum Physics
-
T
Why Are Allowed Energy Levels Odd at ##x=0##?
I know that there is a boundary condition at ##x=0## where the wave function becomes zero however why are the allowed energy levels odd i.e. ##n=1, 3, 5 ..##- tryingtolearn1
- Thread
- Energy Energy levels Levels
- Replies: 11
- Forum: Advanced Physics Homework Help
-
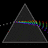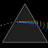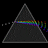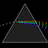
Hydrogen Emission Spectrum and Electron Energy Levels
1. The 4th line from the left, being the aqua blue line, corresponds to a wavelength of 486 nm, as blue light has a wavelength in the range 450-495 nm. 2. This is where I am having the most difficulty, I have tried to answer the question comprehensively but I am not satisfied with my answer. In...- AN630078
- Thread
- Electron Emission Emission spectrum Energy Energy levels Hydrogen Levels Spectrum
- Replies: 2
- Forum: Introductory Physics Homework Help
-
L
High School Basic Question: What are energy levels, of photons, for example?
I often read of photons manifesting different levels of energy. I know that energy increases as wavelength decreases and frequency increases. Are there other ways particles gain or lose energy? As water boils heat energy is transferred to the water causing water molecules to move faster and to...- lazarericus
- Thread
- Energy Energy levels Example Levels Photons
- Replies: 8
- Forum: Quantum Physics
-
S
Calculating degeneracy of the energy levels of a 2D harmonic oscillator
Too dim for this kind of combinatorics. Could anyone refer me to/ explain a general way of approaching these without having to think :D. Thanks.- sukmeov
- Thread
- 2d Degeneracy Energy Energy levels Harmonic Harmonic oscillator Levels Oscillator Quantum
- Replies: 12
- Forum: Advanced Physics Homework Help
-
J
Undergrad Accuracy of the Density of States
I'm trying to understand the detailed concept of why the density of states formula is accurate enough to calculate the number of quantum states of an energy level, including degeneracy, within a small energy interval of ##dE##. The discrete energie levels are calculated by $$E = \frac{h^2 \cdot...- JohnnyGui
- Thread
- Accuracy Degeneracy Density Density of states Energy levels Quantum states States
- Replies: 3
- Forum: Quantum Physics
-

High School Ionization energy and energy levels
Hi, The ionization energy is defined as the minimum amount of energy required to remove the most loosely bound electron, the valence electron, of an isolated neutral gaseous atom. Our physics teacher also told us while explaining this concept in the context of energy levels, that it is equal...- ilovepudding
- Thread
- Energy Energy levels Ionization Ionization energy Levels
- Replies: 11
- Forum: Quantum Physics
-
A
Undergrad Nucleus energy levels, neutron energy
From what I read I see that for example a radioactive nucleus is a nucleus in an excited state and when it transitions back to it's stable state (changes from one element to another due to radioactive decay) one of the ways this happens is that the nucleus emits a energetic photon with a...- artis
- Thread
- Energy Energy levels Levels Neutron Nucleus
- Replies: 15
- Forum: High Energy, Nuclear, Particle Physics
-
S
Degeneracy of hydrogen energy levels
I'm considering a hydrogen atom placed in an infinite potential on one side of the nucleus, i.e. ##V(x) = +\infty## for ##x < 0##. I require the wavefunctions to be odd in order to satisfy the boundary condition at ##x=0##. By parity of the spherical harmonics only states with ##l## odd are...- Silicon-Based
- Thread
- Degeneracy Energy Energy levels Hydrogen Levels Quantum mechanics
- Replies: 1
- Forum: Advanced Physics Homework Help
-

Undergrad Energy levels from the propagator
I would like to get some information on this topic. It is not discussed in many places, so if any members here know about it, i would be interested in a brief explanation. Or any books or online documents where it is discussed. D is the "invariant propagation function" or the "propagator". I...- PGaccount
- Thread
- Energy Energy levels Levels Propagator
- Replies: 1
- Forum: Quantum Physics
-

Internal Energy of a Mole of Particles each with 3 Energy Levels
Hello, I'm doing some refreshers before going back to school. Stat mech is my shakiest and I'd appreciate some help on this problem. I know that for a single particle, the partition function will be $$Z = 1 + 2e^{-\beta\Delta} + 2e^{-4\beta\Delta}$$ and so its internal energy is $$\frac{1}{Z}...- danyull
- Thread
- Energy Energy levels Internal Internal energy Levels Mole Particles
- Replies: 4
- Forum: Advanced Physics Homework Help
-
J
High School Atom's mass -- does it change with energy levels?
Summary: Does an atom in an excited state have a higher mass than when in its ground state? Summary: Does an atom in an excited state have a higher mass than when in its ground state? Does an atom in an excited state have a higher mass than when in its ground state?- jeremyfiennes
- Thread
- Change Energy Energy levels Levels Mass
- Replies: 13
- Forum: Atomic and Condensed Matter
-
C
Can atoms have Vibrational and Rotational energy levels?
I found one answer somewhere else in the internet, It specified there that atoms cannot have rotational and vibrational energies since they don't have a point on them that will allow the atom to be rotated or vibrated. However , that answer did not suffice so I ask the same question here.- CGandC
- Thread
- Atoms Energy Energy level Energy levels Levels Rotational Rotational energy
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
J
Undergrad Electron wave funtion harmonic oscillator
As we see in this Phet simulator, this is only the real part of the wave function, the frequency decreases with the potential, so lose energy as moves away the center. we se this real-imaginary animation in Wikipedia, wave C,D,E,F. Because with less energy, the frequency of quantum wave...- jhonnyS
- Thread
- Electron Energy Energy levels Harmonic Harmonic oscillator Oscilator Oscillator Wave Wave function
- Replies: 2
- Forum: Quantum Physics
-
M
Undergrad Energy Levels: Why Do Spacings Get Smaller as Excitation Increases?
Hi All. For the above energy level diagram, why do the energy levels spacings proceed to get smaller and smaller as the excitation energy increases?- Martin89
- Thread
- Energy Energy levels Levels Nuclear Nuclear energy Nuclear physics Nucleus
- Replies: 7
- Forum: High Energy, Nuclear, Particle Physics
-
Explain the energy levels of Samarium-154
These are the experimental results for the energy levels of Samarium-154: From 0+ to 8+ I recognize rotational energy: $$E = \frac{l(l+1)\hbar^2}{2I}$$ But from there on I do not know how to justify the rest. I have been reading that we sometimes get energy diagrams like above for even-even...- JD_PM
- Thread
- Energy Energy levels Explain Levels
- Replies: 1
- Forum: Advanced Physics Homework Help
-

Medical How Do Hormones and Neurons Trigger the Physiology of Hunger?
I have been studying about the physiology of hunger. I have studied about the hormones that are involved in the process of hunger, like ghrelin, leptin, pancreatic polypeptide, cholecystokinin, cholecystokinin (CCK) and the neurons like AgRP, POMC and neuropeptide Y (NPY) etc. I understand...- mktsgm
- Thread
- Energy Energy levels hormones Levels Neurons Physiology
- Replies: 3
- Forum: Biology and Medical
-
C
Conflict Between Infinite energy levels and Valence Shells
Homework Statement I'm having trouble understanding the existence of valence shells. I understood that valence shell is the last energy level for the electrons to populate around an atom. But, according to Bohr model , an atom can have infinite energy levels , so I don't understand: How can...- CGandC
- Thread
- Bohr model Energy Energy levels Infinite Infinite energy Levels
- Replies: 7
- Forum: Biology and Chemistry Homework Help
-

Graduate Energy requirements for creating water mist
The basis of the question is: in creating a mist that you sprayed on a surface could you get an energy transfer to the surface equivalent to an optical emission in the infrared? (Far Infrared). How much energy is imparted to room temp water in creating a spray of particles around 5 microns... -

Finding the 10 lowest energy levels
Homework Statement Homework Equations The Attempt at a Solution I understand the equation, and I understand the concept. My question is this: What is the best way to go about solving this problem? My line of reasoning concludes that the fourth lowest energy level is E211. However, the...- Mason Smith
- Thread
- Energy Energy levels Levels Modern physics Schrodinger equation
- Replies: 4
- Forum: Introductory Physics Homework Help
-
N
Is this really an excited state?
The figure below is from a textbook. It is explaining what excited states are using carbon as an example. I don't necessarily agree that the the state labeled as "example excited state 1" is really an excited state. Since the electrons in the 2p orbitals are unpaired, and in the absence of a...- nezahualcoyot
- Thread
- Energy levels Excited Excited states Ground state Spin State
- Replies: 2
- Forum: Chemistry
-

High School Why are absorption spectra continuous?
It doesn't make sense to me that absorption spectra are (mostly) continuous. Here are my beliefs. Please tell me which piece/pieces is a/are misconception(s). 1) When light is absorbed, the energy is used to excite an electron to some discrete energy level. 2) To get to this discrete energy...- Cardinalmont
- Thread
- Absorption Absorption spectrum Continuous Emission spectrum Energy levels Spectra
- Replies: 9
- Forum: Atomic and Condensed Matter
-

Graduate Nuclear and Atomic energy levels
I've noticed that in the Shell Model of the nucleus, the order of the energy levels is 1s, 1p3/2, 1p1/2, etc. While in the atomic energy levels it goes 1S 2S 2P, ... But they still take the same amount of particles for each level in both the atomic and nucleus. Am I missing something here? Or...- QuarkDecay
- Thread
- Atom Atomic Energy Energy levels Levels Nuclear Nucleus
- Replies: 3
- Forum: High Energy, Nuclear, Particle Physics
-
S
Undergrad Gamma absorption at increasing energy levels
Which are main absorption modes of matter for gamma rays under about 500 MeV? And how strong is the absorption? For low energies (under 20 MeV) the mechanisms are: 1) Photoelectric absorption - all energies from eV range onwards 2) Compton scattering - all energies enough to displace the...- snorkack
- Thread
- Absorption Energy Energy levels Gamma Increasing Levels
- Replies: 5
- Forum: High Energy, Nuclear, Particle Physics
-
L
Bessel Function Zeros - To find Energy Levels
[Mentors' note: Moved from the technical forums, so no template] Hi, I have to find energy levels of an electron in a cylindrical shape. I know how to derive the formula below: However, I'm not sure which zero value and what intger p I need to use in order to find the lowest energy. If these...- LiorSh
- Thread
- Bessel Bessel function Energy Energy levels Function Levels
- Replies: 9
- Forum: Advanced Physics Homework Help
-

Diagram of energy levels of hydrogen lines
Homework Statement a) Draw a diagram of energy levels to explain the spectrum of lines of the hydrogen atom b) Indicate, for each photon, that its region can be emitted to the electromagnetic spectrum. c) Compare, in a graph, the energies of the orbital of the hydrogen atom with the energies of...- José Ricardo
- Thread
- Diagram Energy Energy levels Hydrogen Levels Lines
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-

Energy levels of a system with just two electrons?
Homework Statement If a system comprised only of two electrons was physically possible (such as positronium but with two electrons), what would its energy levels be and how would they relate to the energy levels of Helium? Homework Equations ##E_{Helium} = E_{n1}+E_{n2}=-\frac{\mu Z^2...- It's me
- Thread
- Electrons Energy Energy levels Helium Identical particles Indistinguishability Levels System
- Replies: 4
- Forum: Advanced Physics Homework Help