Ideal gas Definition and 823 Threads
-
G
Using Ideal Gas Law to Calculate Vertical Pressure Gradient
Homework Statement Consider a cylindrical parcel of air of area A and infinitesimal height dz. If this air parcel is to remain stationary, the difference between the total pressure forces exerted on its top and bottom faces must be equal to its weight. Use this information and the ideal gas...- Ghost Repeater
- Thread
- Air pressure Gas Gas law Gradient Ideal gas Ideal gas law Law Pressure Pressure gradient Vertical
- Replies: 2
- Forum: Calculus and Beyond Homework Help
-
Q
Calculating Mercury Column Height in J-Shaped Tube: Manometry Homework Solution
Homework Statement A J-shaped tube has an uniform cross section and it contains air to atmosphere pression of 75 cmo of Hg. It is pours mercury in the right arm, this Compress the closed air in the left arm. Which is the heigh of the mercury's column in left arm when the right arm is full of...- Queren Suriano
- Thread
- Ideal gas
- Replies: 7
- Forum: Biology and Chemistry Homework Help
-

Undergrad Application of the ideal gas law
Hi, I want to calculate the amount of liquid nitrogen (at boiling temp.) needed to build a pressure of 10.1 bar in a vessel of volume 66 m3. The liquid will be poured slowly into the vessel, boil off and fill the volume with gas at the specified pressure. I make the assumption that the process...- doktorglas
- Thread
- Application Gas Gas law Ideal gas Ideal gas law Law
- Replies: 2
- Forum: Thermodynamics
-
H
Find the change in the Kinetic energy of an Ideal Gas
Homework Statement Let 3/2kT be the kinetic energy of ideal gas per molecules. T the absolute temperature and N the avogadro number. Answer the following questions : 1) when the volume doubled at constant temperature. How many times the kinetic energy per molecule become greater than before...- Helly123
- Thread
- Change Energy Gas Homework physics Ideal gas Ideal gases Kinetic Kinetic energy Thermodaynamics Thermodinamic
- Replies: 39
- Forum: Introductory Physics Homework Help
-

Undergrad How to describe the Sun's interior?
My question to you is this... Can the interior of the Sun be described as an ideal gas? From my knowledge, to describe a body of gas as an ideal gas, the separation between the particles must be much greater than the size of the actual particles. How could one justify whether the Sun fits this?- mjda
- Thread
- Ideal gas Interior Matter Sun
- Replies: 43
- Forum: Astronomy and Astrophysics
-

Undergrad Understanding the Molar Heat Capacity of an Ideal Gas
We know that for an ideal gas the differential of the internal energy function is: dU = n Cv dT But is Cv the molar heat capacity or not?- House
- Thread
- Capacity Gas Heat Heat capacity Ideal gas molar heat capacity Thermodynamics first law
- Replies: 1
- Forum: Thermodynamics
-
H
Heat and work when temperature increases by 1 degree
Homework Statement Kinetic energy per mol is 3/2KTHomework Equations Q = nC##\Delta##T U = Q + W W = -P##\Delta##V The Attempt at a Solution 1) internal energy = 3/2NKT 2) heat needed to increase temperature of 1 mol ideal gas by 1 degree at constant volume? Since constant volume, W = 0 Q =...- Helly123
- Thread
- Degree Heat Ideal gas Ideal gas law Temperature Thermodyamics Work
- Replies: 21
- Forum: Introductory Physics Homework Help
-

Graduate Statistical Mechanics Part II: The Ideal Gas - Comments
Greg Bernhardt submitted a new PF Insights post Statistical Mechanics Part II: The Ideal Gas Continue reading the Original PF Insights Post. -
G
What Is Cv for an Ideal Gas with Cp = 35.4 J/mol⋅K?
Homework Statement If Cp for an ideal gas is 35.4 J/mol⋅K, which of the following is Cv for this gas? a. 12.5 J/mol⋅K b. 20.8 J/mol⋅K c. 29.1 J/mol⋅K d. 27.1 J/mol⋅K e. 43.4 J/mol⋅K Homework Equations ΔH = ΔE + Δ(PV) = Q + W + Δ(PV), and for ideal gas, ΔH = nCvΔT + Δ(nRT) = nCvΔT + nRΔT =...- grangr
- Thread
- Gas Heat Ideal gas Specific Specific heat
- Replies: 10
- Forum: Introductory Physics Homework Help
-
G
Why Might Statement (b) Be Incorrect in Ideal Gas Processes?
Homework Statement Which of the following statement(s) is (are) correct when an ideal gas goes from an initial to a final state in a single process? a. No work is done on or by the gas when the volume remains constant. b. No energy is transferred into or out of the gas as heat transfer when the...- grangr
- Thread
- Gas Ideal gas Thermodynamics
- Replies: 2
- Forum: Introductory Physics Homework Help
-
J
Ideal gas - percentage of fraction of molecules
Homework Statement Homework EquationsThe Attempt at a Solution Honestly speaking , I have absolutely no idea about this problem .This doesn't happen often .In the KTG chapter , only definition and formula of Average , RMS and Most probable speeds is given . This question is for an...- Jahnavi
- Thread
- Fraction Gas Ideal gas Molecules
- Replies: 9
- Forum: Introductory Physics Homework Help
-
T
High School Adiabatic or Isobaric process?
Consider the following problem: Gaseous helium (assumed ideal) filled in a horizontal cylindrical vessel is separated from its surroundings by a massless piston. Both piston and cylinder are thermally insulating. The ambient pressure is suddenly tripled without changing the ambient temperature...- Terry Bing
- Thread
- Adiabatic Ideal gas Process Thermodynamics
- Replies: 61
- Forum: Thermodynamics
-

High School Equations that describe ideal gas processes
Hello! Is there a table to show the equations that describe ideal gas processes? For example, I know for isothermic, it's P1V1=P2V2, what about the others? Also, how are these derived? Is it from Q-W=dU? or PV=nRT? Any help? thanks!- physea
- Thread
- Gas Ideal gas
- Replies: 6
- Forum: Thermodynamics
-

Undergrad Calabi-Yau manifold + ideal gas + point disturbance?
Because it is a closed space, can it make sense to fill a Calabi_Yau manifold with an ideal gas and consider waves from a point disturbance? Would the Ricci-flat condition of Calabi-Yau manifolds have anything to say about possible sound waves? Thanks!- Spinnor
- Thread
- Gas Ideal gas Manifold Point
- Replies: 3
- Forum: Beyond the Standard Models
-

Thermodynamics: Ideal Gas Law, find the temperature
Homework Statement A 3-ft^3 container is filled with 2-lbm of oxygen at a pressure of 80 psia. What is the temperature of the oxygen?Homework Equations pV= nRT T= PV/nR R= 10.7316 psia x ft^3/ lbmol x R The Attempt at a Solution Hi everyone! So I understand how to use the Ideal Gas Law but my...- AbbeyC172
- Thread
- Gas Gas law Ideal gas Ideal gas law Law Temperature Thermodynamics
- Replies: 5
- Forum: Introductory Physics Homework Help
-
H
Find the work done on a monoatomic gas in this P-V diagram
Homework Statement Homework Equations internal change = $$\frac{3}{2}n.R.(T2 - T1)$$ Work = P.ΔV The Attempt at a Solution 1) T2 = $$\frac {P2. V2 . T1 }{P1 . V1} = 1.2 * 10^3$$ 2) Q = Internal change = $$\frac{3}{2}n.R.(T2 - T1) $$ $$ = \frac{3}{2} * 1 *8.3*10^{-3}*(12*10^2 - 3*10^2) $$...- Helly123
- Thread
- Diagram Gas Ideal gas P-v diagram Thermodyamics Work Work done
- Replies: 39
- Forum: Introductory Physics Homework Help
-

Free energy per unit volume of an ideal gas
Homework Statement Homework EquationsThe Attempt at a Solution [/B] I am taking the free energy as the internal energy of the ideal gas. Then the average internal energy per unit volume is ## \frac { 3 nk_B T } {2 } ##. So, the correct option is (c). Is this correct?- Pushoam
- Thread
- Energy Free energy Gas Ideal gas Jnu 2013 Per per unit Thermodyamics Unit Volume
- Replies: 3
- Forum: Introductory Physics Homework Help
-

Energy of a Gas in equilibrium with BB-radiation
Homework Statement A closed, thermally-insulated box contains one mole of an ideal monatomic gas G in thermodynamic equilibrium with blackbody radiation B. The total internal energy of the system is ##U=U_{G}+U_{B}##, where ##U_{G}## and ##U_{B} (\propto T^4)## are the energies of the ideal gas...- VSayantan
- Thread
- Black body radiation Energy Equilibrium Gas Ideal gas Internal energy
- Replies: 9
- Forum: Introductory Physics Homework Help
-
M
Ideal Gas Law Homework: Calculating Number Density and Spacing Between Molecules
Homework Statement Consider an ideal gas at 25.0 degrees Celsius and with a pressure of 1.00 atm. a) What is the "number density" of the molecules, expressed as molecules per unit volume? (Cubic meter, cubic centimeter or liter) b) What is the typical spacing between molecules in the gas? Of...- Matt Armstrong
- Thread
- Gas Gas law Ideal gas Ideal gas law Law
- Replies: 2
- Forum: Introductory Physics Homework Help
-
J
What is the Ideal Gas Law for a Two-Bulb System with Varying Temperatures?
1. Two equal glass bulbs are connected by a narrow tube and the whole is initially filled with a gas at a temperature of T0 and pressure of P0. Then, one of the bulbs is immersed in a bath at a temperature, T1 and the other in a bath at a different temperature, T2. Show that in this problem, the...- Jean2005
- Thread
- Gas Gas law Ideal gas Ideal gas law Law Physcis
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help
-
H
Undergrad Ideal Gas Equation and Temperature change?
During a thermodynamic process, the state variables of an ideal gas, measured in kPa, m3, and oC, varied in the following way: P2 = 2P1 V2 = 3V1 T2 = 8T1 What is the temperature increase of the gas, T2 −T1 ? I've been given an answer of 4780 and I'm not sure how to get there?- HannahJB
- Thread
- Change Gas Ideal gas Temperature Temperature change
- Replies: 6
- Forum: Thermodynamics
-
J
Internal energy of an ideal gas as a function of pressure?
Assuming all gases in the combustion reaction of benzoic acid (C6H5COOH) behave ideally, what is the "exact" change in internal energy? The context in which this question is being asked is after a calorimetry experiment. For all the intents and purposes of calorimetry, the change in internal...- Jacob Dale
- Thread
- Calorimetry Energy Enthalpy Function Gas Ideal gas Internal Internal energy Pressure Thermodaynamics
- Replies: 5
- Forum: Introductory Physics Homework Help
-
H
How to Calculate Ideal Gas State Properties Using Molecular Dynamics?
Dear all, I want to calculate thermodynamical properties of my molecule which I am calculating its thermodynamical properties of non-ideal part using Molecular dynamics. I need ideal gas state total energy, Cp, and Cv in several different temperatures. I am using opt+freq at B3lyp/6-311++G(d,p)...- hosein
- Thread
- Calculation Computational chemistry Gas Ideal gas State Thermodynamcics
- Replies: 2
- Forum: Chemistry
-

Undergrad Internal energy of any ideal gas
The internal energy of monoatomic ideal gas is due to the kinetic energy of the molecules. Using Boltzmann Maxwell distribution, it is calculated that the kinetic energy due to translational motion of gas molecules of an ideal gas depends only on the temperature. In case of monoatomic gas, since...- Pushoam
- Thread
- Energy Gas Ideal gas Internal Internal energy
- Replies: 2
- Forum: Thermodynamics
-
F
Energy of an ideal gas given its multiplicity
Question: The multiplicity of an ideal gas is given by g(U) = A.U3N/2, where U is the energy of the gas, A is a constant and N is the number of particles in the gas. Prove that the energy of the gas given a temperature T is U = (3/2).N.kb.T Attempts: My first thought was to...- felipedc
- Thread
- Energy Gas Ideal gas multiplicity Temperature
- Replies: 1
- Forum: Advanced Physics Homework Help
-
F
Work done pumping air into a bottle
Hi there, i am struggling with the following problem. Air is pumping into a bottle with volume V and pressure Pi until it reaches a final pressure Pf. The temperature remains the same during the process and the gas is an ideal one. We have to calculate the work that is done. I am not quite...- Funky_Sp
- Thread
- Air Ideal gas Work Work done
- Replies: 17
- Forum: Introductory Physics Homework Help
-

Adiabatic Compressor: Ideal Gas Temperature Change
Homework Statement A mixture of hydrogen, carbon monoxide and carbon dioxide is being fed to a compressor at 2.0 bar and 25 C. The overall flow rate is 17.47 SCMS and its composition is 73.5 mol% H2, 13.7 mol% CO and the balance CO2 The compressor operates adiabatically and reversibly with a...- runningman19
- Thread
- Adiabatic Adiabatic compression Change Chemical engineering Compressor Gas Homework Ideal gas Temperature Temperature change
- Replies: 2
- Forum: Engineering and Comp Sci Homework Help
-
E
Internal energy of an ideal gas as a function of temperature
Homework Statement Two containers hold an ideal gas at the same temperature and pressure. Both containers hold the same type of gas but container B has twice the volume of container A. The internal energy of the gas in container B is (a) twice that for container A (b) the same as that for...- eprparadox
- Thread
- Energy Function Gas Ideal gas Internal Internal energy Temperature Thermodynamcics
- Replies: 2
- Forum: Introductory Physics Homework Help
-
L
What is the Effect of Isothermal Movement on Internal Energy of a Gas?
Homework Statement The dot in Fig. 19-18b represents the initial state of a gas, and the isotherm through the dot divides the p-V diagram into regions 1 and 2. For the following processes, determine whether the change Eint in the internal energy of the gas is positive, negative, or zero: (a)...- L_landau
- Thread
- Heat Ideal gas Isothermal Thermodynamics
- Replies: 2
- Forum: Introductory Physics Homework Help
-

Molecular Specific Heat of an Ideal Gas: Computations
Homework Statement A cylinder with a heavy ram/piston contains air at T = 300 K. Pi = 2.00 * 105 Pa, Vi = 0.350 m3, Mr = 28.9 g/mol & Cv = 5R/2 (a) What's the Molecular Specific Heat of an Ideal Gas, with a constant volume, computed at J/KgC ? (Cv) (b) What's the mass of the air inside the...- Const@ntine
- Thread
- Gas Heat Ideal gas Molecular Specific Specific heat
- Replies: 8
- Forum: Introductory Physics Homework Help
-
G
Two gases separated by a piston
Homework Statement [/B] Two ideal gases are contained adiabatically and separated by an insulating, fixed piston that blocks the molecules of gas 2 but allows the molecules of gas 1 through(in both directions). The initial pressures, volumes, temperatures and number of molecules on each side is...- GandalfTheGrey
- Thread
- Gases Ideal gas Piston Thermodyamics
- Replies: 6
- Forum: Introductory Physics Homework Help
-

Undergrad Pressure exerted by an ideal gas
Hi Please have a look on the attachment. Suppose that a 0.1 kg rubber ball having velocity of 60 m/s is moving between two walls A and B, and the distance between the walls is 1 m. It is having elastic collisions with the walls. Let's focus on what is happening at wall B. The ball is moving...- PainterGuy
- Thread
- Gas Ideal gas Pressure
- Replies: 80
- Forum: Mechanics
-
A
Statistical Physics: Quantum ideal gas
Homework Statement I'm reading the book about Statistical Physics from W. Nolting, specifically the chapter about quantum gas. In the case of a classical ideal gas, we can get the state functions with the partition functions of the three ensembles (microcanonical, canonical and grand...- aburriu
- Thread
- Gas Hamiltonian Ideal gas Physics Quantum Quantum mechahnics Statisical physics Statistical Statistical physics
- Replies: 1
- Forum: Advanced Physics Homework Help
-

Measure the volume of a lighter than air balloon
hello, i am trying to calculate the volume of a balloon (which is quite large). It has been filled with helium via a valve connecting helium storage tanks to the balloon. The knowns I have are the volume of the storage tanks, the intital pressure in the tanks, and the final pressure in the tanks...- psuedoben
- Thread
- Air Balloon Ideal gas Measure Volume
- Replies: 3
- Forum: Mechanical Engineering
-
U
Pressure dependence of the equilibrium constant for an ideal gas
I read in some scripts that equilibrium constant for an ideal gas is not a function of pressure: But that is not generally true! Since: $$\left (\frac{\partial \Delta_{R} G}{\partial p} \right )_{T,\vec{n}}=\Delta_{R} V$$ and $$\Delta_{R} G^{0}=-RT\cdot \ln K$$ it should be: $$\left... -

Undergrad Kinetic theory of gases: rebound speed and force questions
Hi everyone, I remember years ago at school memorising the derivation of the formula for pressure in the kinetic theory of gases, as explained in this Youtube video: Thinking a little more deeply about this derivation there are two things I don't get: 1) At 0:53, the video says the molecule...- Amaterasu21
- Thread
- Conservation of momentum Elastic collision Force Gases Ideal gas Kinetic Kinetic theory Rebound Speed Theory
- Replies: 2
- Forum: Mechanics
-

Internal Energy of an Ideal gas related to Molar specific heat
Homework Statement Please look at the below images which is the derivation of the relation between the internal energy of an ideal gas and the molar specific heat at constant volume. (Snaps taken from Fundamentals of Physics Textbook by David Halliday, Jearl Walker, and Robert Resnick) As...- SciencyBoi
- Thread
- Energy Gas Heat Ideal gas Internal Internal energy Kinetic theory of gases Specific Specific heat Thermodynamics
- Replies: 2
- Forum: Introductory Physics Homework Help
-
P
Ideal Gas Law -- Isobaric Epansion followed by....
Homework Statement An ideal gas with Cv = 5/2R, and γ = 1.4 starts at a volume of 1.5m3 , a pressure of 2.0×105Pa, and a temperature of 300K. It undergoes an isobaric expansion until the volume is V , then undergoes an adiabatic expansion until the volume is 6.0m3 , and finally undergoes an...- physics123
- Thread
- Gas Gas law Homework Ideal gas Ideal gas law Introductory physics Law
- Replies: 9
- Forum: Introductory Physics Homework Help
-
M
Internal Energy of virial expansion
Hello, I have some trouble understanding the virial expansion of the ideal gas. 1. Homework Statement I have given the state equation: $$ pV = N k_b T \left(1+\frac{A\left(T\right)}{V}\right) $$Homework Equations [/B] and a hint how to calculate the caloric equation of state $$...- Mikhail_MR
- Thread
- Energy Expansion Ideal gas Internal Internal energy Statistical phyisics
- Replies: 10
- Forum: Advanced Physics Homework Help
-
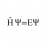
Graduate Ideal gas pressure from Maxwell-Boltzmann distribution
Hi everyone I'm having trouble with solving an exercise in statistical physics. I need to argue why the average number of particles with a velocity between ##v## and ##v+dv## that hit a surface area ##A## on the container wall in a time interval ##\Delta t## is $$N_{collision}=v_{x}A\Delta t...- H Psi equal E Psi
- Thread
- Distribution Gas Ideal gas Maxwell boltzmann Pressure Statistical phyisics Thermodyamics
- Replies: 4
- Forum: Electromagnetism
-

Change of internal energy of an ideal gas
Homework Statement Homework Equations Δu = ∫ [(a-Ru)+bT+cT^2+dT^3]dT The Attempt at a Solution The answer of 6447kJ/kmol is given but I am struggling to get to this answer after integrating the above formula and inserting the given values. Firstly would the integral of...- DevonZA
- Thread
- Change Energy Gas Ideal gas Internal Internal energy
- Replies: 5
- Forum: Introductory Physics Homework Help
-
R
Partition function for ideal gas for "medium" temperature
Looking for the heat capacity of ideal gas due to rotational degrees of freedom. If the temperature of the gas is much higher than the temperature corresponding to the energy differential between states,the partition function can be written as the integral over the density of states. If the...- rockyleg
- Thread
- Function Gas Ideal gas Medium Partition Partition function Temperature
- Replies: 1
- Forum: Introductory Physics Homework Help
-
E
Ideal gas in a cylinder with a piston
Homework Statement A vertical cylinder of radius r contains an ideal gas and is fitted with a piston of mass m that is free to move. The piston and the walls of the cylinder are frictionless, and the entire cylinder is placed in a constant-temperature bath. The outside air pressure is p0. In...- Elias Waranoi
- Thread
- Cylinder Equilibrium Force Gas Ideal gas Piston
- Replies: 5
- Forum: Introductory Physics Homework Help
-
C
Calculating Entropy Change for an Ideal Gas Expansion
MENTOR NOTE: NO TEMPLATE BECAUSE SUMITTED TO WRONG FORUM. 3.1) A quantity of 0.10 mol of an ideal gas A initially at 22.2 degrees C is expanded from 0.200 dm3 to 2.42 dm3 . Calculate the values of work (w), heat (q), internal energy change (delta U), entropy change of the system (deltaSsys)...- chemochem
- Thread
- Change Entropy Expansion Gas Gas expansion Ideal gas
- Replies: 7
- Forum: Biology and Chemistry Homework Help
-
M
MHB Which Gas Behaves Closest to an Ideal Gas?
out of the gasses hydrogen,nitrogen,chlorine, and helium which shows behviour closest to an ideal gas.? ive been told the answer is helium because there is no attractive forces between helium atoms. would there not be van der waal forces though.? also would hydrogen just have as little...- markosheehan
- Thread
- Gas Ideal gas
- Replies: 1
- Forum: General Math
-
M
Thermodynamics: Compression of an Adiabatic Gas
Homework Statement Assume 1.500 mol of a monatomic ideal gas is compressed from 3.00 L to 1.00 L. a. If the initial and final temperature is 10.0 °C, what are the initial and final pressures (in atm)? b. How much work input (in kJ) is required if a reversible isothermal path at 10.0 °C is...- Minescrushessouls
- Thread
- Adiabatic Colorado Compression Gas Ideal gas Thermo Thermodyamics Thermodynamics
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
Z
Pressure Equalization of X & Y Gas Bottles
Homework Statement X and Y are two gas bottles that are connected by a tube that has negligible volume compared with the volume of each bottle. There is a valve in the tube that is initially closed. X has a volume of 2V and contains hydrogen at a pressure of p, Y has a volume V and contains...- zanyzoya
- Thread
- Gas Ideal gas Pressure Tube
- Replies: 10
- Forum: Introductory Physics Homework Help
-
J
Understanding the Different Forms of the Ideal Gas Law and Their Applications
Hey I was hoping someone could be me a succinct method of knowing what form of the Ideal gas law I need to use and in particular the different R's associated with each form. Form my Thermodynamics class we use PV = nRT Pv = RT PV = mRT Little v being the specific volume (which changes the R...- Jack Duncan
- Thread
- Forms Gas Gas law Ideal gas Ideal gas law Law Thermodynamcics
- Replies: 5
- Forum: Chemistry
-
L
Final temperature real gas behaving ideally
Homework Statement Please consider a mixture of oxygen (1 mole), nitrogen (4 mole), and carbon dioxide (3 mole). The mixture was heated in a well - insulated vessel with 753 kJ. Determine the final temperature if the mixture is composed of real gases behaving ideally as described by Eq. 3.48...- Logan McEntire
- Thread
- Final Final temperature Gas Ideal gas Real gas Specific heat Temperature Thermo
- Replies: 2
- Forum: Introductory Physics Homework Help
-

An ideal gas closed system reversible process
Homework Statement an Ideal gas at T = 70 C and 1 bar undergoes following reversible processes: a: Adiabatically compressed to 150 C b: then, cooled from 150 to 70 C at constant pressure c: finally, expanded isothermally to the original state (T=70 C and P = 1 bar) Homework Equations...- HethensEnd25
- Thread
- Closed Closed system Gas Ideal gas Process Reversible Reversible process System
- Replies: 2
- Forum: Biology and Chemistry Homework Help