Ideal gas Definition and 823 Threads
-
P
Problem related to the Ideal Gas equation -- Nitrogen under pressure
Solution from the textbook: My work: I constantly get 1.55kg. I also tried dividing the first and the second equation (pxV=m/M x R x T with different values). How did they come up with the equation in the solution? Also, I am sorry if I posted it in the wrong place and didn't follow the rules...- PhysicsDuki
- Thread
- Gas Ideal gas Nitrogen Pressure
- Replies: 2
- Forum: Introductory Physics Homework Help
-
K
Proof question related to the Ideal Gas Law
A cylinder contains an initial volume V1 = 1m^^3 of a perfect gas at initial pressure p1 = 1 bar, confined by a piston that is held in place by a spring. The gas is heated until its volume is doubled and the final pressure is 5 bar. Assuming that the mass of the piston is negligible and that the...- Kajan thana
- Thread
- Gas Gas law Ideal gas Ideal gas law Law Proof Thermodynamic
- Replies: 8
- Forum: Introductory Physics Homework Help
-

MHB Maths in Temperature and ideal gas Thermometer
Hi, I didn't understand the maths involved in the below article in regard to temperature and ideal gas thermometer. If any member knows it, may reply me. If triple point of water is fixed at 273.16 K, and experiments show that freezing point of air-saturated water is 273.15 K at 1 atm...- WMDhamnekar
- Thread
- Gas Ideal gas Temperature Thermometer
- Replies: 2
- Forum: General Math
-
K
Finding the Volume of an Ideal Gas
Hi, I tried to do this question in two different approaches one of them was using the equation PV=mRT where I got the right answer which is 4.305 m**2. However, I tried using this Density = Mass/Volume, where I substituted Denisity= 1.225 and Mass equals 5kg to get the volume as 4.08. Can...- Kajan thana
- Thread
- Gas Ideal gas Volume
- Replies: 4
- Forum: Introductory Physics Homework Help
-
J
Chemistry Ideal Gas Law and Partial Pressures
I had already found the Mass of the product (C3H3N) produced by this reaction (theoretical mass at 100% yield) in a previous problem. I did this by finding the Limiting Reagent (C3H6) in the reaction , calculating the number of moles of C3H6 and using the Molar Ratios in the balanced reaction...- jackthehat
- Thread
- Gas Gas law Ideal gas Ideal gas law Law Partial
- Replies: 16
- Forum: Biology and Chemistry Homework Help
-
A
Ideal gas: two tanks connected by a cylinder
I ASSUME THAT THE PRESSES OF THE TWO CONTAINERS WILL BE EQUAL IN THE FINAL (STATIONARY REGIME). SO Pa = Pb naRTa/V = nbRTb/V naTa = nbTb Than , I just need to set up a system My question is , so, will the two pressures at the end be the same? And as for the temperatures, can I also say...- A13235378
- Thread
- Cylinder Gas Ideal gas
- Replies: 5
- Forum: Introductory Physics Homework Help
-

Adiabatic expansion of a piston in a cylinder filled with ideal gas
I was puzzling over how to solve this and finally peeked at the solution. They used the relevant equation above. I disagree with this though. The problem specifically says “the piston is allowed to slide freely!” This means that we don’t let it happen slowly. So then we are not in...- Hiero
- Thread
- Adiabatic Adiabatic expansion Cylinder Expansion Gas Ideal gas Piston
- Replies: 19
- Forum: Introductory Physics Homework Help
-
L
Undergrad When does the ideal gas equation break down?
P1/V1/T1 = P2 V2 /T2 is derived from the ideal gas equation. However it is stated that this equation breaks down at very high pressures and at very low temperatures. Does anyone know what kind of pressures and temperatures we are talking about here?- LT Judd
- Thread
- Break Gas Ideal gas
- Replies: 5
- Forum: Thermodynamics
-
E
High School What is the significance of Δx and Δp in the ideal gas entropy equation?
Up to an undetermined constant ##a## the entropy of an ideal gas goes like$$S = k_B N\ln \left[ a^\frac{3}{2} \left(\frac{V}{N}\right) \left(\frac{E}{N}\right)^{\frac{3}{2}} \right]$$In some notes is written: And then they identify ##\Omega = \left(\frac{\Delta x \Delta p}{w}\right)^{3N}##...- etotheipi
- Thread
- Gas Ideal gas Microstates
- Replies: 2
- Forum: Thermodynamics
-

What will be the number of collision per second in a unit area?
By using PV=nRT formula, I have found the volume of the vessel. As far as I have learned to calculate the number of collision in a unit volume. So, it is being difficult for me to find the right way to solve. I searched on the internet and have got this...- Mahfuz_Saim
- Thread
- Area Collision Ideal gas Per Unit
- Replies: 12
- Forum: Introductory Physics Homework Help
-
P
Finding the efficiency of an ideal gas with adiabatic exponent 'γ'
Here is what I did : work done in going from A to C, W1 = 2nRToln(2) (isothermal process) work done in going from C to B, W1 = pΔV = nRΔT = -nRTo (isobaric process) work done in going from B to A, W3 = 0 (isochoric process) so, total work done = W1 + W2 + W3...- PhysicsEnthusiast123
- Thread
- Adiabatic Efficiency Exponent Gas Homework Ideal gas Thermodynamcics
- Replies: 3
- Forum: Introductory Physics Homework Help
-
I
Chemistry What are the partial pressures of each gas in the mixture?
I've first calculated the partial pressures of each gas: ##N_2: 0.4\times 7.4\times 10^4=3.0\times 10^4 Nm^{-2}\\## ##O_2: 0.35\times 7.4\times 10^4=2.6\times 10^4 Nm^{-2}\\## ##CO_2: 0.25\times 7.4\times 10^4=1.9\times 10^4 Nm^{-2}\\## From here, I do not know how to continue. Could someone...- ianc1339
- Thread
- Gas Ideal gas Partial Partial pressure Pressure
- Replies: 16
- Forum: Biology and Chemistry Homework Help
-
I
Question about calculating the minimum temperature in hot air balloons
First, I tried using the Archimedes principle and calculated the weight of the surrounding air displaced when taking off. ##W = 2500\times 1.29\times 9.81 = 31637.25 N## But then, I got stuck and do not know how to proceed from here on. I don't want the full solution yet but can I get some...- ianc1339
- Thread
- Air Archimedes principle Hot Ideal gas Minimum Pressure Temperature
- Replies: 6
- Forum: Introductory Physics Homework Help
-
P
Entropy and the Helmholtz Free Energy of a Mass-Piston System
Attempt at a Solution: Heat Absorbed By The System By the first law of thermodynamics, dU = dQ + dW The system is of fixed volume and therefore mechanically isolated. dW = 0 Therefore dQ = dU The change of energy of the system equals the change of energy of the gas plus the change of energy...- Pendleton
- Thread
- Energy Entropy Free energy Helmholtz Helmholtz free energy Ideal gas Statisical mechanics System Thermodyamics
- Replies: 8
- Forum: Introductory Physics Homework Help
-

Graduate Flaw in alternative approach to determine ideal gas speed distribution
If we assume the energy of particles in an ideal gas follows a Boltzmann distribution, then the energy distribution function can be defined as below: , where k_B is the Boltzmann constant Since the energy of particles in an ideal gas are assumed to only consist of translational kinetic energy...- shadedvertex
- Thread
- Approach Distribution Gas Ideal gas Speed
- Replies: 3
- Forum: Mechanics
-

How is entropy affected in a reversible process for an ideal gas?
What do I see in my solution is : ΔW + ΔQ = W_pv + W' + ΔQ (A little difficult to perceive the useful work ) Work on the environment : -p0*(-ΔV) (WHY negative sign?, Is this the work ON the gas?) ΔV=nR (T0/p0 -T/p) By TdS = dQ ΔS + ΔS0 =0 Reversible case: ΔU= -T0ΔS - (-p0(-ΔV)) + W' (WHY...- Pouyan
- Thread
- Gas Ideal gas Work
- Replies: 3
- Forum: Advanced Physics Homework Help
-

Speed of sound in an ideal gas
First of all I thought it was necessary to calculate the temperature(the only data missing for the formula) using the ideal gas equation(since I've already been given 'p' and 'V'), and plug it in the 'v' formula, but the problem immediately occurred when i tried to find out the number of...- greg_rack
- Thread
- Gas Ideal gas Sound Speed Speed of sound
- Replies: 6
- Forum: Introductory Physics Homework Help
-
D
Accounting for Liquid Water Density in Ideal Gas Pressure Calculations
First calculate the total pressure, but that gives me p2=p1*t2/t1 = 2,86 bar and partial pressure of 0,87 bar which is wrong. any tips?- dbag123
- Thread
- Gas Ideal gas Pressure
- Replies: 6
- Forum: Introductory Physics Homework Help
-

Ideal gas in a cylindrical container
It looks more like a computational obstacle, but here we go. Plugging all of these to the partition function: $$Q = \frac{1}{N! h^{3N}} \int -\exp(\frac{1}{2m}(p^2_{r}+p^2_{\phi}/r^2+p^2_{z})+gz)d\Gamma=$$ $$= \frac{1}{N! h^{3N}} \int \exp{(\frac{-1}{2m}p^2_{r})}dp_{r_{1}}...dp_{r_{N}}...- CptXray
- Thread
- Container Cylindrical Gas Ideal gas Statistical mechanics
- Replies: 4
- Forum: Advanced Physics Homework Help
-

Pressure of an ideal gas -- A Level Multiple Choice Problem
- maxelcat
- Thread
- A level Choice Gas Ideal gas Multiple Multiple choice Pressure
- Replies: 2
- Forum: Introductory Physics Homework Help
-
F
Moving an adiabatic partition in an adiabatic container
The actual data for the problem and my (and my friend's) attempt at a solution are in the attached file. In a nutshell, this is what happened. I obtained a solution based on the fact that the system is isolated. Thus the initially hot gas moves the partition doing work onto the initially cold...- FranzDiCoccio
- Thread
- Adiabatic Adiabatic process Container Ideal gas Partition
- Replies: 49
- Forum: Introductory Physics Homework Help
-

General solution of the spherical wave equation
Since the spherical wave equation is linear, the general solution is a summation of all normal modes. To find the particular solution for a given value of i, we can try using the method of separation of variables. $$ ψ(r,t)=R(r)T(t)ψ(r,t)=R(r)T(t) $$ Plug this separable solution into the...- ContagiousKnowledge
- Thread
- 3d General General solution Ideal gas Oscillation Spherical Wave Wave equation
- Replies: 20
- Forum: Advanced Physics Homework Help
-
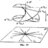
Ideal Gas Law: Question about a compressor exam question
This is a question in my midterm. I calculated for the answer as c) 11.7 atm by the Ideal Gas Law. The professor states that "all the air is originally at 1 atm" in the prompt indicates an idea of "both 70 L of air and existing 6 L of air in the tank are at 1 atm", and he grades d) 12.7 atm as...- Zifan Wang
- Thread
- Compressor Exam Gas Gas law Ideal gas Ideal gas law Law
- Replies: 4
- Forum: Introductory Physics Homework Help
-
B
Ideal gas: Temperture at 1 atm
Summary: U=3/2*n*R*T Can some of you help me with this The total internal energy of an ideal gas is 3770 J. If there are 3 moles of the gas at 1 atm, what is the temperature of the gas? I use U=3/2*n*R*T but get the wrong answer, (101 K) but it should be 303 K [Moderator's note: Moved from...- Baronen
- Thread
- Gas Ideal gas Tempeature
- Replies: 2
- Forum: Introductory Physics Homework Help
-
M
Chemistry Need Help with Ideal Gas Problem?
Hello guys. I really try to understand the problem, can you please help.- mimimycin
- Thread
- Chemistry Gas Ideal gas
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
N
Ideal Gas Law in Two Dimensions
I am creating a two-dimensional model of an ideal gas, and I was wondering how I should determine initial velocity. Ideally, I would like for the simulation to reach a point where the velocity distribution resembles that of the maxwell-boltzmann curve — will this be achieved if I, say, assign... -
S
Changing the Air Temperature with a hair dryer
Hi, so I found this on another old "AP" High School Finals Exam. I think I may be super lost. Because the only way that I can think about is KE = 3/2kT. And then that the difference of the Kinetic Energy of the air Particles is the Q supplied by the heater inside the air dryer. So ## \frac...- spsch
- Thread
- Air Hair Ideal gas Temperature Thermodynamics
- Replies: 24
- Forum: Introductory Physics Homework Help
-

Calculations using the Ideal Gas Law
2.1 * 10^-4m/3 Temperature 310K Pressure: 5.3 * 105 Pa So the Ideal gas formula is PV = nRT 2.1*10^-4m^3 Times 5.3*105Pa = n * Gas Constant * Temperature 2.1*10^-4m^3 (*) 5.3*105Pa = # of moles * I'm not sure what I was doing, but the whole equation stuff got hard and I stopped. I left...- ArcHorizon
- Thread
- Calculations Gas Gas law Ideal gas Ideal gas law Law
- Replies: 2
- Forum: Introductory Physics Homework Help
-
J
Undergrad How Do You Calculate Initial Pressure and Final Volume in Adiabatic Expansion?
I have a compressed pure gas at a specific temperature and volume. (T1, V1) It suddenly (adiabatically) expands until it's at ambient pressure and a specific temperature. (P2, T2). Given: T1, V1, T2, and P2, I want to find P1 and V2. There's a great example in wikipedia which is almost...- JeffEvarts
- Thread
- Adiabatic Adiabatic expansion Expansion Ideal gas Pressure Temperature Volume
- Replies: 7
- Forum: Thermodynamics
-

Undergrad KT in systems other than ideal gas
Hi All, When dealing with the kinetic theory of gases in thermodynamics, we obtain the result that the mean kinetic energy per atom is (3/2) kT. In considering different samples like 200g of liquid water or a solid cube of lead with one cubic meter, does kT still play an important role in...- DaTario
- Thread
- Gas Ideal gas Systems
- Replies: 8
- Forum: Thermodynamics
-

Find Vol 2 in Ideal Gas Law Problem with V1 and V2 Open and V3 Shut
Please refer to diagram. V1 is open initially then V2 is open for 5 minutes for pressure to equalize. V1 and V2 are then shut. V3 is opened. What is Vol 2 ? P(final)*V(final) = n(final)* R*T => (Vol1 + Vol2) = n(final)*R*25C/ 0.070 Torr where n(final) = n(Vol1) + n(Vol2) If I shut V3, I...- Catstranaughts
- Thread
- Gas Gas law Ideal gas Ideal gas law Law
- Replies: 6
- Forum: Introductory Physics Homework Help
-

Graduate Can the isothermal expansion of an ideal gas be irreversible?
For the reversible expansion of an ideal gas the heat flowing out of the surroundings and into the system is equal to the work done by the system. Since both system and surroundings have the same constant temperature the entropy increase of the system is equal to the entropy decrease of the...- Philip Koeck
- Thread
- Expansion Gas Ideal gas Irreversible Isothermal
- Replies: 5
- Forum: Thermodynamics
-

Ideal gas problem after constraint removed
Attempt: P_1 (initial pressure on the left section) P_2(initial pressure on the right section) T_f, P_f (final pressure for both sections) P_1 (V/3) = N/2 k (3T/2) P_2 (2V/3) = N/2 k (T/2) P_f V/2 = N/2 k T_f Resulting in 4 unknowns and 3 equations... Not enough to find T_f...- Clara Chung
- Thread
- Constraint Gas Ideal gas
- Replies: 1
- Forum: Introductory Physics Homework Help
-
R
Undergrad Thermodynamics and ideal gas law concepts
I'm having trouble wrapping my head around some thermodynamics and ideal gas law concepts. I don't have a specific textbook question but Just a concept I'm having trouble with. What I'm struggling with is understanding some of the relations between pressure, volume and temperature...- ryley
- Thread
- Concepts Gas Gas law Ideal gas Ideal gas law Law Thermodynamics
- Replies: 14
- Forum: Thermodynamics
-

Fluid Dynamics - Mass Conservation, State Equation for an Ideal Gas
I understand that ##\dot m=\rho Q## and ##{\dot m}_{in}= {\dot m}_{out}## . So one can say that ##\rho Q_1 = \rho Q_2##. But I'm not sure if that equation is correct. I don't know if the density remains constant, or the volume flow rate. And then how I'm also supposed to tie a state equation in...- WhiteWolf98
- Thread
- Conservation Conservation of mass Dynamics Equation of state Fluid Fluid dynamic Fluid dynamics Gas Ideal gas Mass Mass conservation State
- Replies: 4
- Forum: Introductory Physics Homework Help
-
T
How Is Internal Energy Calculated for an Ideal Gas Using Temperature Change?
I have the definition of change in internal energy. $$ \Delta U = Q - W $$ I can get the work by $$ W = \int_{V_1}^{V_2} p dV = p \Delta V $$ however the pressure isn't constant so this won't do. ## W ## is work done by the gas and ## Q ## is amount of heat energy brought into the system. I'm...- tuki
- Thread
- Energy Gas Ideal gas Internal Internal energy Work
- Replies: 1
- Forum: Introductory Physics Homework Help
-
L
High School Confused about the ideal gas law
Ok, i am struggling to figure something out. I don't know why math is so much easier than physics haha. ok, here is my struggle. I have two states, state 1 and 2, which i will call just 1 and 2. 1: T=298kelvin V=0.025m(cubed) P=310Kpa Mass1=Mass2 R=0.2870 2: T=323kelvin V=0.025m(cubed) P=...- LT72884
- Thread
- Confused Gas Gas law Ideal gas Ideal gas law Law
- Replies: 16
- Forum: Thermodynamics
-

Minimum heat removed from gas to restore its state
Homework Statement After a free expansion to quadruple its volume, a mole of ideal diatomic gas is compressed back to its original volume isobarically and then cooled down to its original temperature. What is the minimum heat removed from the gas in the final step to restoring its state...- hnnhcmmngs
- Thread
- Free expansion Gas Heat Ideal gas Minimum State Thermodyamics
- Replies: 5
- Forum: Introductory Physics Homework Help
-
T
Is the Ideal Gas Law Valid for Balloon Problems?
Homework Statement Homework Equations Ideal gas law The Attempt at a Solution The solution to this problem assumes the pressure inside the balloon is the same as the outside pressure, i.e. atmospheric pressure. Is this a valid assumption? I would guess otherwise.- Taulant Sholla
- Thread
- Archimedes principle Balloon Gas Gas law Ideal gas Ideal gas law Law Moles Thermodynamics
- Replies: 5
- Forum: Introductory Physics Homework Help
-

What would be a real-life example of the ideal gas law?
Homework Statement What is a real-life example of the ideal gas law? Homework Equations PV = nRT (Pressure x volume = number of moles x the gas constant x temperature in Kelvin) The Attempt at a Solution https://www.reference.com/science/ideal-gas-law-used-everyday-life-3dacbd6ebd3b5949...- Lolaamaigatti04
- Thread
- Example Gas Gas law Ideal gas Ideal gas law Law
- Replies: 3
- Forum: Introductory Physics Homework Help
-
A
Undergrad Free expansion of an ideal gas
If considering the free expansion of an ideal gas adibatically such that the final volume is double of the initial volume. Since dU=0 for free expansion ,which implies Ti=Tf for ideal gas...(1) From adiabatic relation , TiViγ-1=TfVfγ-1, to satisfy (1) ,γ-1=0 and for ideal gas Cp-Cv=nR or...- Apashanka
- Thread
- Expansion Free expansion Gas Ideal gas
- Replies: 10
- Forum: Thermodynamics
-
S
Ideal Gas Law and Pressure at 80°C
Homework Statement An ideal gas has a molar mass of 40 g and a density of 1.2 kg m-3 at 80°C. What is its pressure at that temperature? Homework Equations PV=nRT R constant= 8.314 n= number of moles T= tempreture in kelvin density=Mass/ Volume The Attempt at a Solution i simply solved it like...- SakuRERE
- Thread
- Gas Gas law Ideal gas Ideal gas law Law Pressure
- Replies: 2
- Forum: Introductory Physics Homework Help
-

How Many Balloons Can Be Inflated from a Helium Cylinder?
Hi everyone, I'd really appreciate any help with this problem: A helium cylinder for the inflation of party balloons hold s 25.0L of gas and is filled to a pressure of 16500kPa at 15 degrees celsius. How many balloons can be inflated from a single cylinder at 30 degrees celsius if the volume of...- relatively-uncertain
- Thread
- Gas Ideal gas Introductory physics Universal
- Replies: 16
- Forum: Introductory Physics Homework Help
-
A
Undergrad Specific heat at constt. volume of an ideal gas
The ans comes out (c) if I take specific heat at constt volume to be independent of temp. Whether the specific heat is always temp. independent for an ideal gas??- Apashanka
- Thread
- Gas Heat Ideal gas Specific Specific heat Volume
- Replies: 1
- Forum: Thermodynamics
-
T
Thermodynamics of a Single-Component Ideal Gas
Homework Statement In all calculations, take R = 8.31 J/m-K. Use the Sackur-Tetrode equation with N replaced by n and K replaced by R to calculate the changes in entropy. Also, assume that these processes are quasi-static so that the ideal gas law and the first law apply at all times. Consider...- TRB8985
- Thread
- Gas Ideal gas Thermodynamics
- Replies: 1
- Forum: Introductory Physics Homework Help
-

Undergrad What is the difference between a perfect gas and an ideal gas?
What is difference between perfect gas and ideal gas?- Death eater
- Thread
- Difference Gas Ideal gas Perfect gas
- Replies: 18
- Forum: Thermodynamics
-
R
Undergrad Density of states in the ideal gas
The MB energy distribution is: MB_PDF(E, T) = 2*sqrt(E/pi) * 1/(kB*T)^(3/2) * e^(-E/(kB*T)) How do I arrive at the density of states which hides inside the expression 2*sqrt(E/pi) * 1/(kB*T)^(3/2) ? I've only seen DOS that have "h" in them.. I want it to contain only E, pi, kB and T.. This is...- rabbed
- Thread
- Density Density of states Gas Ideal gas States
- Replies: 2
- Forum: Thermodynamics
-
Basic physics units problems involving the Ideal Gas Law
Homework Statement The following is the equation of ideal gas law, where p is pressure (Force/Area), V is volume, n is number of moles and T is temperature in Kelvin. What is the fundamental unit of R? pV = nRT A. kg^−1 · m^−2 · s^ 2 · K · mol B. kg^−1 · m^−4 · s ^2 · K · mol C. kg · m^4 · s...- jamiebean
- Thread
- Basic physics Gas Gas law Ideal gas Ideal gas law Law Physics Units
- Replies: 5
- Forum: Introductory Physics Homework Help
-
T
Undergrad Confusion about the work done by an ideal gas
When an ideal gas,in a piston kind of system and whose equilibrium state is mentioned, is allowed to expand (piston is allowed to move and not gas leaking )against a constant external pressure very quickly, then, is the work done by gas zero or not zero ? The argument for work being zero is...- theblackfish
- Thread
- Confusion Gas Ideal gas Thermal physics Thermodynamics Thermodynamics first law Work Work done
- Replies: 5
- Forum: Mechanics
-
S
Canonical partition function of an ideal gas (unit analysis)
Homework Statement Basically the units of the Canonical Partition Function within the logarithms should be zero Homework Equations The Attempt at a Solution N here is a number so we ignore the left logarithms, applying a "Unit function " for the terms within the logarithm...- Somali_Physicist
- Thread
- Analysis Function Gas Ideal gas Partition Partition function
- Replies: 2
- Forum: Advanced Physics Homework Help