rey242
- 40
- 0
Homework Statement
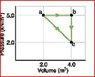
One mole of an ideal diatomic gas undergoes a transition from. a to c along the diagonal path
What is the change in internal energy?
Homework Equations
The Attempt at a Solution
I've tried to use the two other paths since the internal energy is the same both ways, but I can't see anyway to reconcile the two.