Latent heat Definition and 199 Threads
-
P
Latent Heat Problem? Not sure how to solve
Homework Statement A window of a car is found to be covered with 0.8cm of ice. If the area of the window is 1 m2, and the ice is at -12 degrees Celsius, what is the minimum heat required to melt all the ice? Take the density of ice to be 900 kg/m3, the specific heat capacity of ice to be 0.5...- PBryan833
- Thread
- Heat Latent heat Phase change
- Replies: 3
- Forum: Introductory Physics Homework Help
-
J
Undergrad Latent Heat of Fusion & Internal Energy
I understand the main concepts around this topic but I am struggling to find any information about the potential energy involved in the latent heat of fusion. My book defines internal energy as kinetic and potential unlike an ideal gas that is assumed to have just kinetic energy. When a...- Jimmy87
- Thread
- Energy Fusion Heat Internal Internal energy Latent heat
- Replies: 2
- Forum: Thermodynamics
-
M
How do I find the specific latent heat of fusion of nitrogen?
When doing an experiment to find the specific latent heat of fusion of liquid nitrogen, you find that 100 kJ of heat must be removed to freeze 3.8kg of nitrogen at its freezing point. What value will you get for the specific latent heat of fusion of nitrogen? Power=100kj M=3.8kg I don't...- max1020
- Thread
- Fusion Heat Latent heat Nitrogen Specific
- Replies: 3
- Forum: Introductory Physics Homework Help
-
G
Graduate How to estimate latent heat of polymorphic phase transitions
Hello, I know that the latent heat of a first-order isothermal and isobaric phase transition is in general given by the difference in enthalpies between the two phases, but my question is: do you know if there is there a way to estimate its value "from first principles" for the case of a...- g_mogni
- Thread
- Estimate Heat Latent heat Phase Phase transitions
- Replies: 3
- Forum: Thermodynamics
-
S
Latent Heat of Fusion and Ice Maker Problem
Homework Statement You are required to calculate the efficiency of an ice-making machine that takes in water at a temperature of 16°C and produces ice cubes at a temperature of -6°C. Water is taken in at the rate of 1kg every 5 minutes and the input power to the machine is 300W. The Specific...- Silent
- Thread
- Fusion Heat Ice Latent heat
- Replies: 8
- Forum: Engineering and Comp Sci Homework Help
-
R
Thermal Conductivity and latent heat
Homework Statement If a copper kettle has a base of thickness 2.0mm and an area 3.0 x 10-2 m2 estimate the steady difference in temperature between the inner and outer surface of the base which must be maintained to enable enough heat too pass through so that the temperature of 1 kg of...- risingabove
- Thread
- Conductivity Heat Latent heat Thermal Thermal conductivity
- Replies: 3
- Forum: Introductory Physics Homework Help
-
B
Latent Heat Question: Calculate Vaporization Rate & Energy Needed
Homework Statement A scientist heats up a substance by applying heat at a constant rate of 75 J/s. She measures that it takes 1 minute for 100 grams of the substance to evaporate. What is the latent of vaporization of this substance? If she starts with 1 kg of the substance, how long...- Bgerst103
- Thread
- Heat Latent heat
- Replies: 3
- Forum: Introductory Physics Homework Help
-
V
Length of cooling including latent heat
Hello, I would like to know how to calculate how long the phase transition when cooling lasts. So basically how long the latent transition lasts. I really don't have any ideas how this could be calculated, although I would assume it is function of the mass. Any help is much appreciated.- VoorMMMMMM
- Thread
- Cooling Heat Latent heat Length
- Replies: 1
- Forum: Introductory Physics Homework Help
-

What is the role of ε in understanding latent heat?
This is a question of understanding notes today:) I'm struggling to follow these notes, they are not very well written and a tad sporadic! I was just reading through them and I'm not quite sure what the term ε actually is in this case? I am assuming it is a constant although on the first page...- KiNGGeexD
- Thread
- Heat Latent heat
- Replies: 1
- Forum: Introductory Physics Homework Help
-
F
Undergrad Does this equation exist? (Latent heat, temperature, entropy)
Evening all. I've seen this equation written down but can not for the life of me find it on tinterweb, is it correct? L = Tconst(S2-S1)- Flucky
- Thread
- Entropy Heat Latent heat Temperature
- Replies: 2
- Forum: Thermodynamics
-
P
Latent heat of Fusion and transfer problem
Homework Statement A 100g (.1kg) cube o ice at 0dC (d=degrees) is placed in 1kg of water that was originally at 80dC. What is the nal temperature of the water after the ice has melted? Answer: 65.5dCHomework Equations Lf = Q/m Q=(DelT)mCThe Attempt at a Solution At first I looked up the latent...- PhysicsStuff
- Thread
- Fusion Heat Latent heat
- Replies: 6
- Forum: Introductory Physics Homework Help
-
F
Thermal physics - entropy change (latent heat, specific heat etc)
Hi all, could somebody have a look over my answers for this question please? The value I got for the second part seems quite feeble. Homework Statement The Attempt at a Solution [SIZE="4"]Part a) Key m = mass cs = specific heat bronze cm = specific heat molten bronze Tfus...- Flucky
- Thread
- Change Entropy Heat Latent heat Physics Specific Specific heat Thermal Thermal physics
- Replies: 2
- Forum: Introductory Physics Homework Help
-
B
How Does Latent Heat Affect Sweat Evaporation in Cooling the Body?
The latent heat of vapourisation of H2O at body temperature (37°C) is 2.42x106 J Kg-1. To cool the body of a 75 Kg jogger (average specific heat capacity = 3500 J Kg-1C°-1) by 1.5 °C, how many Kilograms of water in the form of sweat have to be evaporated? Q = mcΔT I'm confused about what to do...- BOAS
- Thread
- Heat Latent heat
- Replies: 3
- Forum: Introductory Physics Homework Help
-
C
High School Latent heat in water vapor below boiling
Hi - I'm a physics newbie so this is an elementary question, but I can't find the answer with a google search. Water evaporates at a wide range of temperatures, what is the latent heat in the water vapor (in calories or Joules), for example, at 30°C, 60°C? Assume standard atmospheric...- ceilidhdad
- Thread
- Boiling Heat Latent heat Vapor Water Water vapor
- Replies: 1
- Forum: Thermodynamics
-
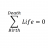
Yes, your approach and calculations look correct. Good job!
Homework Statement How much heat is required to heat 0.2kg of ice from -20°C to 30°C? Homework Equations Total heat lost or gained : ##Q = mcΔt## where ##Δt## is the change in temperature and c is the heat capacity. Latent Heat Fusion : ##Q = mL_f## The Attempt at a Solution From my...- STEMucator
- Thread
- Fusion Heat Latent heat
- Replies: 5
- Forum: Introductory Physics Homework Help
-

Finding latent heat of vaporisation as a function of temperature
Homework Statement The vapour pressure of a certain liquid is given by the equation: \log_{10}P=3.54595-\frac{313.7}{T}+1.40655\log_{10}T where ##P## is the vapour pressure in mm and T is temperature in kelvin. Determine the molar latent heat of vapourisation as a function of temperature...- Saitama
- Thread
- Function Heat Latent heat Temperature Vaporisation
- Replies: 9
- Forum: Biology and Chemistry Homework Help
-
R
Calculating Heat Energy Changes in a Water and Ice System
Homework Statement mass of cup 1.78g initial mass of water and cup - 54.26g finlal mass of water after ice added - 91.26g initial temperature - 27°C temperature after heated - 92°C temperature after ice added - 35°C problem 1) how much heat energy did the original mass of water lose...- Ravi Ramdoolar
- Thread
- Fusion Heat Latent heat
- Replies: 6
- Forum: Introductory Physics Homework Help
-

How to Calculate Latent Heat at a New Point on the Coexistence Curve?
Homework Statement I'm stuck in the following problem: In a particular solid-liquid phase transition the point ##T_0##, ##P_0## lies on the coexistence curve. The latent heat of vaporization at this point is ##l_0##. A nearby point on the existence curve has pressure ##P_0+p## and temperature...- fluidistic
- Thread
- Heat Latent heat
- Replies: 3
- Forum: Advanced Physics Homework Help
-
J
Latent Heat of CAS No. 4719-04-4
I'm hoping someone can help me find information on this, I've been searching to no avail for it. I need to know the latent heat of 2, 2', 2" (Hexahydro-1,3,5-triazine-1,3,5-triyl)triethanol CAS No. 4719-04-4 at P = 480 Psig, T = 150°F. Can anyone point me in the right direction for some info...- jaredogden
- Thread
- Cas Heat Latent heat
- Replies: 3
- Forum: Chemistry
-
J
What is the Difference Between Latent Heat and Specific Heat?
Homework Statement I took a test and got it back I'm wondering if my prof. definition is correct. The heat required per unit mass of a substance to produce a phase change is called? A. specific heat B. Specific heat capacity C. latent heat I said it was C. He say's B Please...- Jbreezy
- Thread
- Heat Latent heat Specific Specific heat
- Replies: 10
- Forum: Introductory Physics Homework Help
-
C
High School What is the definition of latent heat and how does it apply to phase changes?
I need some clarifications on the precise definitions and scope of latent heat. My textbook defines latent heat as the amount of energy absorbed or releases during a phase change process. So if I have a pot of liquid water and I vaporize it completely, then the latent heat is simply the energy I...- cdot
- Thread
- Heat Latent heat Thermodynamics
- Replies: 1
- Forum: Thermodynamics
-
W
Latent Heat Transfer Rate in a Pipeline help please
Hey guys, first post. I'm trying to determine to the rate at which liquids drop out of a gas pipeline. I've modeled the system in a chemical process program, but its only a steady state model. Anyways, here's some of what I can get out of that program: Heat of vaporization Total Heat Transfer...- wsabol
- Thread
- Heat Heat transfer Latent heat Rate
- Replies: 1
- Forum: Mechanical Engineering
-
M
Chemical potential in Thermodynamics to find latent heat of vaporization
Homework Statement The latent heat of vaporization of a substance is defined as the amount of heat required to transform the substance from its liquid into its vapor phase. A certain material has a boiling point of 700K and its molecules have three degrees of freedom in the liquid phase but...- musicure
- Thread
- Chemical Chemical potential Heat Latent heat Potential Thermodynamics Vaporization
- Replies: 7
- Forum: Introductory Physics Homework Help
-
R
Latent Heat and conservation of energy
Homework Statement A cooking vessel on a slow burner contains 11.0 kg of water and an unknown mass of ice in equilibrium at 0°C at time t = 0. The temperature of the mixture is measured at various times. During the first 50.0 min, the mixture remains at 0°C. From 50.0 min to 60.0 min, the...- rico22
- Thread
- Conservation Conservation of energy Energy Heat Latent heat
- Replies: 6
- Forum: Introductory Physics Homework Help
-
L
Experiement of determining specifc latent heat of vapourisation
Homework Statement Explain why heat loss is the same in both cases, provided the time is the same. Homework Equations VIt = mL + H The Attempt at a Solution Is it the same because in both cases I'm asked to boil off 50g of alcohol?- lionely
- Thread
- Heat Latent heat
- Replies: 10
- Forum: Introductory Physics Homework Help
-
Y
Latent Heat + Specific Heat Problem
Homework Statement Suppose molten (liquid) lead, mass ml = 9.83 kg, is at its melting point. The lead is poured into water of mass mw = 585 g and initial temperature Tw = 16.2 C. Find mwb, the amount of the water that boils. NOTE: Reaching the boiling point is not enough; the question asks...- yaylee
- Thread
- Heat Latent heat Specific Specific heat
- Replies: 3
- Forum: Introductory Physics Homework Help
-
D
Calculating the Initial Height of a Hailstone for Complete Melting on Impact
Homework Statement Assume that a hailstone at 0◦C falls through air at a uniform temperature of 0◦C and lands on a sidewalk also at this temperature. From what initial height must the hailstone fall in order to entirely melt on impact? The acceleration due to gravity is 9.8 m/sec2 and the...- DLH112
- Thread
- Heat Latent heat
- Replies: 6
- Forum: Introductory Physics Homework Help
-
W
How Much Ice Remains When Water and Ice Reach Equilibrium in a Styrofoam Cup?
A styrofoam cup contains 2.00x10^2 g of water at 21.0°C. A student adds 60.0g of ice at 0°C into this cup. Determine the mass of ice in the cup when the ice-water mixture reaches equilibrium. Qcold = -Qhot no idea where to start- Wamjam
- Thread
- Heat Latent heat Stoichiometry
- Replies: 1
- Forum: Introductory Physics Homework Help
-
P
Temperature of resistor in latent heat experiment.
Hi, I have conducted an experiment for finding the latent heat of liquid nitrogen using a 5W power supply and a 200 ohms resistor. As part of my conclusions I am asked to discuss/refer to the resistor's temperature during the experiment. I am not sure whether I am expected to provide a...- peripatein
- Thread
- Experiment Heat Latent heat Resistor Temperature
- Replies: 11
- Forum: Introductory Physics Homework Help
-
N
Graduate Relationship between Latent Heat of Fusion and Temperature
Does latent heat of fusion typically go up or down with temperature? I'm trying to calculate the amount of pressure needed to move the melting point of Thorium Dioxide up by 444 degrees kelvin using the Clausius-Clapeyron Law to see whether it would be feasible to line the combustion chamber of...- nlieb
- Thread
- Fusion Heat Latent heat Relationship Temperature
- Replies: 1
- Forum: Thermodynamics
-
A
Heat and phase change: latent heat
Homework Statement A 42kg block of ice at 0°C is sliding on a horizontal surface. the initial speed of the ice is 7.3 m/s and the final speed is 3.5m/s. Assume that the part of the block that melts has a very small mass and that all the heat generated by kinetic friction goes into the block...- alaa410
- Thread
- Change Heat Latent heat Phase Phase change
- Replies: 4
- Forum: Introductory Physics Homework Help
-
K
Finding the specific latent heat of fusion of an ice cube
Homework Statement We completed a lab with the following information Substance Mass(g) Starting temperature (°C) Final Temperature (°C) Ice cube 15.55 0 12 Water 151.25 21 12 Now...- kiro484
- Thread
- Cube Fusion Heat Ice Latent heat Specific
- Replies: 1
- Forum: Introductory Physics Homework Help
-
M
High School Enthelpy of Vaporization vs. Latent heat of vaporization
I am having confusion with the difference between these two, or are they synonymous terms?- member 392791
- Thread
- Heat Latent heat Vaporization
- Replies: 5
- Forum: Thermodynamics
-
L
Latent heat of vaporisation question
Homework Statement A pupil performing an experiment finds that, when the heat supply is 16W, it takes 30 mins for the temp of the water to rise from 20°C to 100°C, and that the rate of evap is very slow even at the latter temp. Estimate an upper limit to the value of the heat capacity of the...- lionely
- Thread
- Heat Latent heat Vaporisation
- Replies: 2
- Forum: Introductory Physics Homework Help
-
S
How long to melt the ice(heat, heat transfer, latent heat)
The graph is in the attachment! ( don't have to download to view the file)Homework Statement 49a) A 0.25 kg piece of ice is warmed by an electric heater and the following graph of temperature is produced. Assume that there has been no loss of energy to surroundings. How much additional time...- supernova1203
- Thread
- Heat Heat transfer Latent heat
- Replies: 3
- Forum: Introductory Physics Homework Help
-
S
Changes of state , latent heat and heat transfers
Homework Statement What is the total amount of heat required to heat a 0.2 kg of ice from a temperature of -20 degrees to a temperature of 30 degrees Celsius Homework Equations Q=mcΔt Q=mLf Q=mLv The Attempt at a Solution To solve this they break it down, almost like a...- supernova1203
- Thread
- Heat Latent heat State
- Replies: 11
- Forum: Introductory Physics Homework Help
-
K
Latent heat and final temperature
Homework Statement An aluminum container whose mass is 205g contains 300g of water at 20°C. In this container is then placed 250g of iron at 150°C and 20g of ice at -10°C. Find the final temperature of the mixture. Given: mAl=205g mH2O=300g TAl1=TH2O1=20°C mFe=250g mice=20g TFe=150°C...- kimkibun
- Thread
- Final Final temperature Heat Latent heat Temperature
- Replies: 4
- Forum: Introductory Physics Homework Help
-
S
Latent Heat and phase changes (difficult)
Homework Statement 10g of ice at 0 degrees celsius is combined with 2 g of steam at 100 degrees celsius. Find the final temperature of the resulting water. Homework Equations mc(Tf- Ti) m(Lf) or m (Li) The Attempt at a Solution ΔQice=mLice+mcwaterTf...- ScienceGeek24
- Thread
- Heat Latent heat Phase
- Replies: 1
- Forum: Introductory Physics Homework Help
-
M
Latent heat, liquid-gas transition, stat mech with gravity
Problem solved.Homework Statement The boiling point of a certain liquid is 95OC at the top of a mountain and 105OC at the bottom. Its latent heat is 1000 cal/mole. Calculate the height of mountain. It's Q4 of chapter 15 in Statistical Mechanics by S.K. MaHomework Equations it should be the...- mchan1014
- Thread
- Gravity Heat Latent heat Stat mech Transition
- Replies: 3
- Forum: Advanced Physics Homework Help
-
H
Latent Heat (enthelpy) of Fusion changes with Pressure?
If the melting point depends on the pressure, does the latent heat of fusion also depend on the pressure? The equation relating the entropy of fusion to the enthalpy of fusion is given by: \Delta H=T\Delta S where T is, apparently, the temperature of melting. So, if the melting point... -
H
How Do You Calculate the Final Temperature of Ice Tea Mixing Hot Water and Ice?
Homework Statement 215 cm3 of hot tea at 95°C are poured into a very thin paper cup with 40 g of crushed ice at 0°C. Calculate the final temperature of the "ice tea". (Hint: think about two processes: melting the ice into liquid and, maybe, warming the liquid.) Homework Equations 0 =...- Herbertus
- Thread
- Capacity Heat Heat capacity Latent heat
- Replies: 1
- Forum: Introductory Physics Homework Help
-
P
Latent Heat, Some Density, and God knows what else
I've been having trouble understanding this second semester of physics thus far. I've done SHM pretty well, but this stuff is just strange to me. So here are the problems verbatum, but i'll fill out the template below to help ease the pains. For problem 1: Homework Statement The heating...- platachog
- Thread
- Density Heat Latent heat
- Replies: 5
- Forum: Introductory Physics Homework Help
-
S
Undergrad Latent heat vaporization vs fusion
Why is the latent heat of vaporisation greater than that of fusion(melting)? I mean in liquid state,atoms are already far apart, so it must require less energy to make them gas(far apart) as forces(electric in nature) between molecules decrease with distance.- SandeshPhy
- Thread
- Fusion Heat Latent heat Vaporization
- Replies: 3
- Forum: Thermodynamics
-
L
Calculating Latent Heat of Vaporisation: 3 kW Kettle, 2.0 kg Water @ 100oC
A 3 kW kettle contains 2.0 kg of water at a temperature close to 100oC. Latent heat of vaporisation for water: Lv=2256 (kJ kg^-1) Q= Lv x mass Ok I understand this problem because I now the answer but I don't understand the process. Like my teacher wrote 2256x10^3 why he wrote...- luigihs
- Thread
- Heat Heat equation Latent heat
- Replies: 7
- Forum: Introductory Physics Homework Help
-
S
Determining latent heat of fusion of ice
Homework Statement For the problem below I was able to work out part (i) but I am stuck on working out part (ii) I tried using information given but was unsuccessful. Can anyone help me out? An insulated copper container of mass 0.250 kg contains 0.350 kg water. Both the container and the...- savva
- Thread
- Fusion Heat Ice Latent heat
- Replies: 10
- Forum: Introductory Physics Homework Help
-
P
Question related to latent heat of fusion of ice
Homework Statement one gram of ice at 0 degree C contracts in volume by 0.091cc on melting. 9gm of a metalis heated to 200 degree C and dropped into and ice calorimeter. The decrease in volume was found to be 0.0182 cc. Calculate the Sp. heat of the metal (ANS:0.09cal/gmC) Homework...- pcsx22
- Thread
- Fusion Heat Ice Latent heat
- Replies: 1
- Forum: Introductory Physics Homework Help
-
F
Specific & Latent Heat - Heat required to turn water in Aluminum Tray -> Ice
Specific & Latent Heat - Heat required to turn water in Aluminum Tray --> Ice Homework Statement 200 g Water (l) is contained in an Aluminum ice tray that has a mass of 340 g. Both is at 18°C. How much heat (Q) must be removed to turn the water into ice at -15°C? Aluminum m[FONT="Times...- format1998
- Thread
- Aluminum Heat Ice Latent heat Specific Water
- Replies: 9
- Forum: Introductory Physics Homework Help
-
F
How Much Heat is Needed to Vaporize Ethyl Alcohol from -50°C to Boiling Point?
Homework Statement How much heat is required to change 1.75 L of Ethyl Alcohol (C2H6O) at -50.0°C to a gas at its boiling point? Ethyl Alcohol V = 1.75 L Ti = -50°C 46 g/mol density = 0.789 g/cm3 boiling point = 78°C specific heat (c)= 2400 J/kg*C° Heat of Vaporization (Lv) =...- format1998
- Thread
- Heat Latent heat Specific
- Replies: 3
- Forum: Introductory Physics Homework Help
-
W
Undergrad Latent Heat of Vaporization /w Temperature
Hi, I would like to ask, why is it that the specific latent heat of vaporization of water at, say 10 degrees Celsius, is considerably higher that at 100 degrees Celsius? It would be great if you could provide an explanation from the molecular view. Thanks.- worwhite
- Thread
- Heat Latent heat Temperature Vaporization
- Replies: 3
- Forum: Thermodynamics
-
P
Specific Heat of Nickel / Latent Heat of Fusion
Homework Statement I've done 2 experiments, one was to find the specific heat of nickel and the other was to find the latent heat of fusion. For the nickel experiment, it involves heating the nickel in a test tube in a water bath, and then transferring the pellets to a Styrofoam cup. With...- paperdoll
- Thread
- Fusion Heat Latent heat Nickel Specific Specific heat
- Replies: 9
- Forum: Introductory Physics Homework Help