tsaitea
- 19
- 0
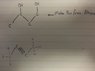
I have attached a file of the alcohol I need to synthesize and my futile attempt... I was thinking of using a hydration reaction to make the alcohol from the alkyne group. So basically I would mix the alkyne group with H2O and some how produce the alcohol group.
Am I heading in the right direction?
Am I heading in the right direction?