Frequently Made Errors in Climate Science – The Greenhouse Effect
Table of Contents
1.What is meant by “The Greenhouse Effect”?
Many gases, such as H2O, CO2, CH4, are transparent to visible light but absorb and emit parts of the infrared spectrum. Most of the visible light reaching the Earth’s surface gets re-emitted, eventually, as infrared. Media that pass visible light through but block infrared can act as heat traps.
2. Real Greenhouses
X “The Greenhouse Effect does not exist; Prof R.W.Wood proved it in 1909.”
Most glass also blocks parts of the infrared band. It was widely believed that this was primarily responsible for the effectiveness of greenhouses.
Prof. Wood suspected that blocking convection was the primary mechanism, so set up a simple experiment to test this. There are a number of weaknesses in the experiment, but the essential conclusion is correct: real greenhouses work primarily by blocking convection.
✓ “Whether or not the Greenhouse Effect exists, it is not the main way real greenhouses work”
However, this just means that the term ‘greenhouse effect’ may be misleading. Wood’s result says nothing about how the atmosphere works.
3. Black Body Earth
If we treat the Earth as emitting and absorbing radiation as a “black body”, ignoring the atmosphere, and treating incoming light as spread evenly over the whole Earth’s surface all the time, we can calculate the equilibrium temperature as -18C. At that temperature, black body radiation would balance insolation.
Adding a non-greenhouse atmosphere, e.g. pure nitrogen doesn’t change this. The atmosphere would take no part in the energy balance. By conduction, it would come to match the surface temperature of the Earth, throughout its depth.
Note:
- With a non-greenhouse atmosphere but now allowing the realities of a rotating sphere, convection would boost the upper atmosphere to something approaching the temperature of the hottest spot on the surface. The surface layer of the atmosphere would be a little cooler by virtue of conduction back to the cooler surface regions.)
- The non-greenhouse atmosphere may also result in some attenuation through Rayleigh scattering. The nitrogen in Earth’s atmosphere may scatter about 4% of light power back into space, taking the temperature down by maybe 3K.
4. The Troposphere
The atmosphere has many layers, featuring quite different processes and temperature profiles.
The atmospheric convection with which we’re familiar only operates up to the tropopause, the top of the troposphere – the band where weather happens. Beyond that, temperature inversions inhibit convection.
X “The ‘greenhouse gases’ are a net coolant since convection carries the heat through the troposphere, past 80% of them. They then block reradiation back to the surface.”
It’s the 20% above the tropopause that matters. This makes the tropopause warmer and/or higher. Since convection is limited by the lapse rate, a higher or warmer tropopause leads to a correspondingly warmer surface.
The existence of this “tropospheric hotspot” is considered a fingerprint of Global Warming.
5. Temperature and Pressure
X “It’s hotter at lower altitudes because it’s at a higher pressure, and compressing a gas heats it”
Compressing a gas heats it, but won’t keep it hot. If the atmosphere were just a static layer of gases, only heated or cooled by conduction, it would all come to the same temperature.
6. The Lapse Rate
X “It’s hotter at lower altitudes because if air rises it expands and cools”
This only explains why convection cannot bring the troposphere to a uniform temperature. It does not explain why there should be a temperature difference in the first place.
Since conduction is not limited by the pressure gradient, there must be an active process producing the temperature gradient. This process is the heating of the Earth’s surface by the sun.
The full story of energy transfers is quite complex. See Trenberth and Kiehl, 1997, Fig 7. Omitting all the absorptions and reradiations:
- The Sun warms the Earth’s surface
- The heat energy is transferred back to the air in the troposphere by a mix of conduction, convection and radiation. Overall, 60% makes it up through the troposphere at least partly by convection, 40% by radiation only.
- Convection’s ability to carry up the heat is limited because of the pressure gradient: rising air expands and cools. The resulting temperature gradient is known as the Lapse Rate
- The troposphere is the layer in which convection can operate. At the top (the tropopause), the temperature gradient is insufficient.
X “We can calculate the surface temperature from the height of the tropopause, the temperature there and the lapse rate. This fixes the surface temperature.”
That has causality backward.
✓ If the mean surface temperature changes, the height of the tropopause will change.
7. Greenhouse Gases
X “There’s nothing special about CO2. All gases can absorb heat”
All gases can conduct heat, but the ability of a molecular species to absorb and emit radiation depends on the intervals in its internal energy states and the polarity of its structure.
If vibration of the atoms in a molecule does not involve a net oscillation of electric field then that vibration cannot absorb or emit electromagnetic radiation. Diatomic gases like N2 and O2 are non-polar (or”homo-polar”). A vibration in the bond between the atoms does not result in any net movement of charge. Other energy levels of those molecules do not have the right intervals to interact with light in either the visible or infrared bands, so are completely transparent to both. Only much higher energies, sufficient to ionize the gases, would be strongly absorbed.
Both water and CO2 are hetero-polar, so can act as dipoles. Some vibrations involve a negatively charged atom group moving one way while a positively charged group moves the other. This net oscillation of charge allows them to interact with radiation at certain frequencies.
X “CO2 is insignificant compared with H2O as a greenhouse gas”
H2O, CO2, CH4 and many others can absorb/emit in parts of the infrared. None of them do so in the entire infrared band. Increasing the level of relatively rare greenhouse gas has more effect than increasing the level of a more common species.
8. Forcings and feedbacks
Climate scientists distinguish factors affecting global temperature as either forcings or feedbacks.
X “Atmospheric H2O is a forcing that overwhelms CO2”
A feedback is a variable which both affects temperature and is affected by temperature. Pure feedback would be a variable entirely controlled by temperature.
These can be further divided into negative and positive feedbacks. This list is by no means exhaustive.
- A few positive feedbacks
- Atmospheric H2O: the warmer the atmosphere, the more water vapor it will hold.
- Polar albedo: as ice caps melt, less incoming light is reflected straight back through the atmosphere.
- The warming of soils and oceans can lead to the release of CO2.
- A negative feedback
- The hotter the Earth’s surface, the more infrared it emits
Note: If something is a negative feedback it acts to dampen change; it cannot make the change go in reverse. That said, delayed feedback can lead to cyclic and chaotic behaviours.
A pure forcing is a variable which affects temperature but is not affected by it. Radiative output from the Sun clearly fits that description.
More loosely, a variable tends to be called a feedback if it is primarily controlled by temperature, and forcing if primarily controlled by other factors. On that basis, anthropogenic CO2 is forcing, but H2O is a feedback.
✓ Anthropogenic CO2 is a significant force because its effect is amplified by positive feedbacks, such as water vapor.
9. The Greenhouse Effect is Logarithmic, roughly
“The light passing through a filter should fall as the negative exponential of the optical thickness, so why is the effect logarithmic?”
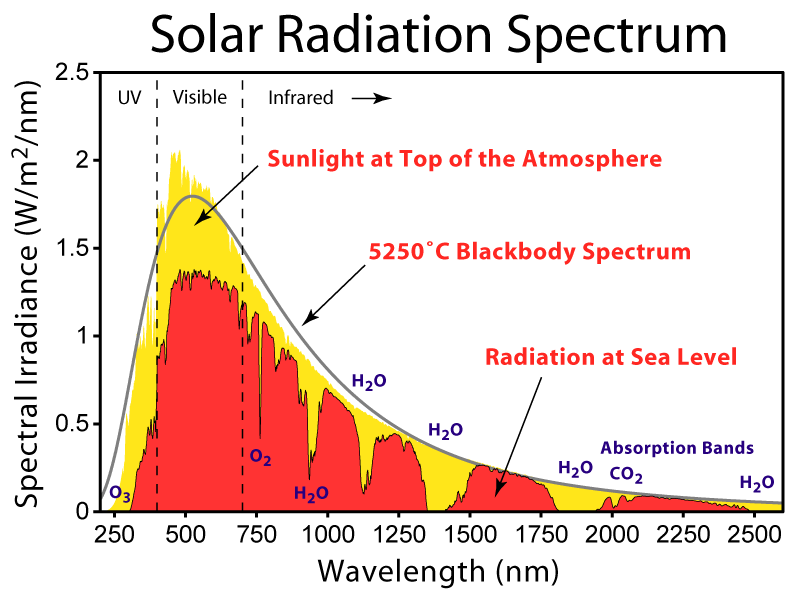
“Solar Spectrum”. Licensed under CC BY-SA 3.0 via Wikimedia Commons – http://commons.wikimedia.org/wiki/File:Solar_Spectrum.png#/media/File:Solar_Spectrum.png; but note the 5250C is incorrect, it is closer to 5777K.
The diagram shows the absorption bands (yellow) for incoming radiation, and the same applies to outgoing. It shows that for most of the width of an H2O or CO2 band, absorption is already substantial.
The quantum basis for specific bands suggests, at first sight, that the wavelengths should be quite precise. However, some subtler processes spread the bands. In particular, the Doppler effect means that molecules moving towards the radiation absorb at a shorter wavelength, while those moving away absorb at a longer.
The primary consequence of adding more of an already abundant gas is to increase the number of molecules at the most extreme speeds relative to the radiation. This increases absorption at the edges of the band, broadening the band slightly. It is this that grows logarithmically with the prevalence of the gas.
“How can it be logarithmic? That would mean adding the first little bit of a new gas would have an infinite effect.”
The logarithmic relationship would not apply for a rarer gas that is still well short of full absorption in the center of its bands. Likewise, it breaks down when bands broaden so much that they overlap.
Masters in Mathematics. Interests: climate change & renewable energy; travel; cycling, bushwalking; mathematical puzzles and paradoxes, Azed crosswords, bridge



Leave a Reply
Want to join the discussion?Feel free to contribute!