Heat Definition and 1000 Threads
-

Heat transfer through a cylindrical shell
Homework Statement An infinitely long cylindrical shell has an inner radius a and outer radius b. If the inside is maintained at a temperature Ta and the outside at a temperature Tb, determine the rate of heat flow per unit length between inner and outer surfaces assuming the shell has a...- Ian Baughman
- Thread
- Conduction Cylindrical Heat Heat transfer Shell
- Replies: 3
- Forum: Introductory Physics Homework Help
-
Z
Heat flux analysis with transient heat conduction
Homework Statement I have one dimensional heating system. In the center is heating source which is heating two PVC elements located on both sides of the heating source. Heat distribution in other dimensions is negligible because of insulation. Thickness of one PVC element is 0,051 m and surface...- Zaffax
- Thread
- Analysis Conduction Flux Heat Heat conduction Heat flux Transient
- Replies: 1
- Forum: Advanced Physics Homework Help
-

Heat exchange in an isolated system
Homework Statement In a container of negligible mass, equal amounts (in weight) of ice at 0°C and steam at 100°C are mixed at atmospheric pressure. Assuming no heat exchange with the surroundings, what is the temperature when the system reaches equilibrium? What are the fractions of weights of...- Ian Baughman
- Thread
- Exchange Heat Heat and thermodynamics Heat conduction Heat exchange Isolated system Physics System Thermodyamics
- Replies: 3
- Forum: Introductory Physics Homework Help
-
L
Undergrad A simple question about reversible heat
Is it possible to heat a specific amount of liquid water, say 1kg, (at 1 atm), let's say from 20°C to 21°C, without changing the universe's entropy? (Sorry but I have a little blackout on simple thermodynamics...) -- lightarrow- lightarrow
- Thread
- Heat Reversible
- Replies: 7
- Forum: Thermodynamics
-
M
Undergrad PDE Heat Equation Solution with Homogenous Boundary Conditions | PF Discussion
Hi PF! I'm wondering if my solution is correct. The PDE is ##h_t = h_{zz}## subject to ##h_z(0,t)=0##, ##h(1,t)=-1##, and let's not worry about the initial condition now. To solve I want homogenous boundary conditions, so let's set ##v = h+1##. Then we have the following: ##v_t = v_{zz}##...- member 428835
- Thread
- Heat Pde
- Replies: 6
- Forum: Differential Equations
-
A
Finding Specific Heat of Unknown Metal
Homework Statement I'm asked to find the specific heat of Metal X in this simulation: http://group.chem.iastate.edu/Greenbowe/sections/projectfolder/flashfiles/thermochem/heat_metal.html Mass of Metal X = 120 g Temp of Metal X = 220 degrees Mass of Water = 30 g Initial Temp of Water = 20...- a1234
- Thread
- Heat Specific Specific heat Temperature
- Replies: 3
- Forum: Introductory Physics Homework Help
-

Watts to heat in soldering iron tip
Hello, I have problem. I'm haveing my soldering iron tips oxidize very fast. I need to know what wattage to use to get melting point of solder. I think I'm overheating my tips. The melting point of the solder I have is 221C (430F). Is there a formula to calculate temperature from wattage input...- IsVictor
- Thread
- Heat Iron Soldering Watts
- Replies: 3
- Forum: Electrical Engineering
-

Plot Heat Capacity vs Temperature for a 2 state system microcananonical ensemble
Homework Statement I have ##C= NK_B (\frac{\epsilon}{K_B T})^{2}e^{\frac{\epsilon}{K_B T}}\frac{1}{(e^{\frac{\epsilon}{K_BT}}+1)^2} ## and need to sketch ##C## vs. ##T## Homework Equations See above The Attempt at a Solution I have ##C= NK_B (\frac{\epsilon}{K_B...- binbagsss
- Thread
- Capacity Ensemble Heat Heat capacity Plot State System Temperature
- Replies: 4
- Forum: Advanced Physics Homework Help
-
A
Undergrad Determining the total energy released by a compost pile
The heat source for this experiment is a compost pile (1 cubic meter), I know that the inside of the pile will reach approximately 50C for 2 weeks or so. Right now it's winter so the temperature will stay around 0C (average). The thermal conductivity of compost can vary greatly but in this case...- AamsterC2
- Thread
- Energy Energy released Heat Temperature Total energy
- Replies: 1
- Forum: Thermodynamics
-
A
Undergrad Determining the energy released by a flame
I've found a lot about using water to find the amount of heat released by something but the experiment I want to run would take about two weeks to complete so that's probably not a viable solution for me. I know what the temperature will be and that it will remain mostly constant, is there...- AamsterC2
- Thread
- Energy Energy released Heat Temperature
- Replies: 17
- Forum: Thermodynamics
-
Z
How to Calculate Steady-State Heat Transfer through a Hollow Sphere?
Hello everyone. I've been doing a bit of independent study for this topic without much background and so my thermodynamic knowledge is fairly limited. I came across this problem and I'd like some assistance with it! If anyone can help out, I would be very grateful. A lot of the equations came...- Zulumike
- Thread
- Heat Heat transfer Sphere
- Replies: 5
- Forum: Advanced Physics Homework Help
-
S
Work and heat transfer in internal combustion engine
Homework Statement : [/B]At the beginning of the compression stroke of a two-cylinder internal combustion engine the air is at a pressure of 101.325 kPa. Compression redeuces the volume to 1/5 of its original volume, and the law of compression is given by PV^1.2 = const. If the bore and stroke...- Suman_babai
- Thread
- Combustion Engine Heat Heat transfer Internal Work
- Replies: 5
- Forum: Engineering and Comp Sci Homework Help
-
E
Graduate How can an adiabatic process decrease internal energy without transferring heat?
I have a dilemma, which has been nagging at me for a week. So first off, can anyone verify my definitions are correct? Heat is the flow of energy, and that flow is caused by the collisions of the atoms in a system. The collisions cause a transfer of KE, hence heat will flow from a substance...- Ehden
- Thread
- Adiabatic Confusion Heat
- Replies: 10
- Forum: Thermodynamics
-

Otto cycle combustion heat question
I have been trying to determine the efficiency of an ideal Otto cycle based on the compression ratio of my car and the heat of combustion of gasoline and I think I'm not entierly wrong but there is something quite off. When trying to calculate the temperature of the combustion stage my result...- Saharka
- Thread
- Combustion Cycle Heat Heat and thermodynamics Otto Specific heat Temperature Thermodynamics
- Replies: 4
- Forum: Mechanical Engineering
-

Undergrad Heat transfer by radiation and Temperature difference
Hello all, this is related to a project that I am working on. It is not directly related to the project but as part of it, I thought it would be a good idea to check the temperature difference that I need to maintain in order to effectively transfer a certain amount of heat between the TH in a...- FQVBSina_Jesse
- Thread
- Difference Heat Heat transfer Radiation Temperature
- Replies: 2
- Forum: Thermodynamics
-
S
Undergrad Physics of Heat Transfer in Glass Windows
What is the physics behind heat transfer between two panes of glass? Commonly windows now are filled with argon (some cost) or krypton (pricey) At a given temperature all gas molecules have the same energy per mode, so heavy ideal gas molecules move more slowly than light ones, so heat...- Sherwood Botsford
- Thread
- Glass Heat Heat and mass transfer Heat transfer Material science Physics Thermodyamics Windows
- Replies: 6
- Forum: Thermodynamics
-
K
Undergrad PDE, heat equation lambda =,<,> 0 question
So I have been studying solving separation of variable, heat equation and came across 2 set of lambda equation. and lambda = 0 have the same equation. Is it different? -
N
Can We Create Coolness Without Heat Using Innovative Technologies?
ok, we have a really efficient way to make heat (adding energy into a system) all we have to do is pass a current through a high resistance wire, boom, close to %100 heat generation. however, from what I can tell there is no way to create coolness (take energy away from a system) like that. we...- NickPerry
- Thread
- Cooling Heat
- Replies: 7
- Forum: Materials and Chemical Engineering
-
A
Undergrad Calculation of heat loss by Degree C/minute.
Hi i was thinking about how to calculate the temperature lost by Degree Celsius per minute and was wondering if you had the surface area of the object, its initial temperature, a constant flow rate of air around the object and probably also need some coefficient of heat loss based on the...- aiop
- Thread
- Calculation Degree Heat Heat loss Loss
- Replies: 1
- Forum: Thermodynamics
-
D
Graduate Electromagnetic Radiation temperature
Is there a meaningful way to convert the energy of an electromagnetic wave to a temperature? I mean this more along the lines of how the universe has a temperature of 2.7 kelvin due to electromagnetic radiation. I'm honestly just curious to determine the temperature of the universe after nearly...- Df241
- Thread
- Electromagnetic Electromagnetic radiation Hawking radiation Heat Heat death Radiation Temperature
- Replies: 2
- Forum: Electromagnetism
-

Stopping door heat loss with curtain
I got an original side door from 1922. Needless to say it's all warped and dented around the sides. My best attempts to weatherize have failed. It's extremely drafty. Various weather strips have not worked and the storm door helps very marginally. I was about to give up and just plastic wrap it...- Greg Bernhardt
- Thread
- Heat Heat loss Loss
- Replies: 28
- Forum: DIY Projects
-
E
Calculating Local Heat Flux in a Pipe: Is h = Nu*(k/x) the Correct Formula?
I am a little unsure how to get started with a homework question. Essentially, I have to calculate the local heat flux at a distance 1.2m (x) along a pipe. I have the fluid's properties and have calculated the Reynolds number, for which I've determined the flow to be turbulent and therefore do...- evoke1l1
- Thread
- Flux Heat Heat flux Heat transfer Local Pipe
- Replies: 2
- Forum: Engineering and Comp Sci Homework Help
-
G
Undergrad Resistance: Dissipated power in collision model
Hi. A simple model explains resistance in metals with collisions of the electrons with the stationary atomic cores. So I assume more collisions result in a higher resistance? But for the dissipated power we have ##P=U^2/R## , which is large for small resistance. I have difficulties combining...- greypilgrim
- Thread
- Collision Dissipation Energy Heat Model Power Resistance
- Replies: 5
- Forum: Electromagnetism
-

Thermodynamics adding ice to water problem with latent heat
Homework Statement [SIZE=13px][FONT=Helvetica Neue]Initially you have mW = 4.6 kg of water at TW = 74°C in an insulated container. You add ice at TI = -19°C to the container and the mix reaches a final, equilibrium temperature of Tf = 33°C. The specific heats of ice and water are cI = 2.10×103...- MattNguyen
- Thread
- Heat Ice Latent heat Thermodynamics Water
- Replies: 1
- Forum: Introductory Physics Homework Help
-
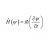
Sublimation: invariant heat or internal energy?
Homework Statement Below, two experiments (1 and 2) are described, in which the same quantity of solid carbon dioxide is completely sublimated, at 25ºC: The process is carried out in a hermetically sealed container, non-deformable with rigid walls; The process is carried out in a cilinder...- Vitor Pimenta
- Thread
- Energy Heat Internal Internal energy Invariant Sublimation
- Replies: 8
- Forum: Introductory Physics Homework Help
-
L
Heat exchanger and cooling towers design
I was wondering all the assumptions I need to make regarding a design for a heat exchanger and cooling tower design from a IC engine testbed. Each engines average output is 55kW and the water jacket temperature is 80 degrees (C) and has to be cooled to 65 degrees (C). The ambient atmospheric...- Lazius
- Thread
- Cooling Design Heat Heat exchanger Mechanical engineering Thermodynamics
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help
-
L
Heat transfer between water tank and pipe system
Hello All, I have a question for heat exchange calculation and hope someone can help. I would like to recover the "cooling energy" from a ice melting pool for air-conditioning for a small office. I plan to install three fan coil units with 800CFM for the office with closed loop water pipe to...- Leo Lee
- Thread
- Heat Heat transfer Pipe System Tank Water Water tank
- Replies: 5
- Forum: Mechanical Engineering
-

When to use C(subv) and C(subp) for Q heat equation
Homework Statement A player bounces a basketball on the floor, compressing it to 80.5% of its original volume. The air (assume it is essentially N2 gas) inside the ball is originally at a temperature of 20.5°C and a pressure of 1.80 atm. The ball's diameter is 23.9 cm. By how much does the...- nso09
- Thread
- Heat Heat equation
- Replies: 2
- Forum: Introductory Physics Homework Help
-

High School Can You Calculate Heat Conductivity with These Simple Variables?
Is there an easy way to calculate the temperature on one side of a material if we know what material it is, the thickness of it and the temperature on the other side. Basically can we calculated the heat transferred through a material if heated. Thank you- TheAnt
- Thread
- Conductivity Heat
- Replies: 6
- Forum: Thermodynamics
-
J
Undergrad Isobaric vs. isothermal expansion
We have a piston with ideal gas in it and a weight. The weight is placed on the piston. The gas is heated externally and the gas expands. Will the expansion be isobaric or isothermal? One argument would be: the expansion will be isobaric because the weight is providing constant pressure. The...- Jaggis
- Thread
- Expansion Heat Ideal gas Isothermal Piston
- Replies: 11
- Forum: Thermodynamics
-
T
High School Can an object ever fully reach ambient temperature in an isolated environment?
I left my unopened icy cold Coke out on my desk yesterday. When I came in today it had, of course, lost it's icy edge and had achieved room temperature. Then I asked myself...will my drink ever actually achieve room temperature or will it always be slightly colder then room temperature? Can...- thetexan
- Thread
- Heat Heat transfer
- Replies: 5
- Forum: Thermodynamics
-

How to Solve the Time Dependent 1D Heat Equation Using Crank-Nicolson Method?
Homework Statement Solve the time dependent 1D heat equation using the Crank-Nicolson method. The conditions are a interval of length L=1, initial distribution of temperature is u(x,0) = 2-1.5x+sin(pi*x) and the temperature in the ends of the interval are u(0,t) = 2; u(1,t) = 0.5. Homework...- apgt512
- Thread
- Computational physics Finite difference Heat Heat equation Time Time dependent
- Replies: 6
- Forum: Engineering and Comp Sci Homework Help
-
J
Conservation of Energy with heat
Homework Statement A 45 kg steel ball is projected vertically with an initial speed of 280 m s . While the ball is rising, 8.5E5 J of heat energy are produced due to air friction. What is the maximum height reached by the ball? Homework Equations Ek = 1/2mv^2 Ep = mgh The Attempt at...- jakeginobi
- Thread
- Conservation Conservation of energy Energy Heat
- Replies: 5
- Forum: Introductory Physics Homework Help
-

Loss of kinetic energy due to heat
Homework Statement I have a bloc sitting on a horizontal table, and we shoot a ball through it. The speed right before entering the block is v and the speed when it exits the block is v/2. I need to prove that the fraction of the initial energy that is lost due to heat is 3/4 - γ/4, where γ is...- Cocoleia
- Thread
- Energy Heat Heat loss Kinetic Kinetic energy Loss
- Replies: 25
- Forum: Introductory Physics Homework Help
-
R
Calculating Heat Transfer in a Cylinder with CO2 Gas at Different Temperatures
Homework Statement Can you please help me with this thermodynamic question?I am not a student. An aluminum cylinder with an interior area of 4.1875 sq ft (or 603 sq in) contains 7.445 lbs of CO2 gas under pressure.The volume of the cylinder is .582 cu ft.The temperature of this gas is 110 deg...- rancam
- Thread
- Heat Heat transfer
- Replies: 3
- Forum: Introductory Physics Homework Help
-
Can flat iron be heated by friction?
< Mentor Note -- thread moved to HH from the technical engineering forums, so no HH Template is shown >[/color] Hi! We are looking for alternative ways to heat up flat iron without using electricty or fuel or solar panel. So we came up with friction (as it is also a source of heat). But we...- Erish
- Thread
- Flat Friction Heat Iron
- Replies: 13
- Forum: Introductory Physics Homework Help
-

Heat Balance Homework: Find Final Water Temp
Homework Statement In heat impervious bowl with unknown volume there are unknown amount amount of water(10 degress celsius). Metal ball with unknown mass / volume / specific heat(ball temperature was 100 degress celsius) - was landed in water and managed to raise temperature to 40 degress...- Ugnius
- Thread
- Balance Heat Heat balance
- Replies: 18
- Forum: Introductory Physics Homework Help
-

Reducing Radiation Heat from Induction Furnace on Lifting Magnet
http://lookpic.com/O/i2/73/DYktKEGg.jpeg Hello Dear Friends We produce Lifting Electromagnet for Steel Scrap charging of Induction furnace, bottom plate of electromagnet is made of Stainless steel . my question : Can We use AL OR SILVER Spray for Spraying bottom plate that Radiation heat...- dormo715
- Thread
- Heat Induction Lifting Magnet Radiation
- Replies: 2
- Forum: Mechanical Engineering
-

What heat capacity is needed to evaporate water in oil
Homework Statement In an industrial fryer is f.e. 400 kW. Heating capacity installed. The fryer may contain as much as 1.450 ltr of oil. I can fry 2.000 kg of chicken nuggets per hour in that. These products loose 7% of moisture/water in this process. That water can only escape from oil in gas...- Adrianus
- Thread
- Capacity Evaporation Heat Heat capacity Oil Steam Water
- Replies: 3
- Forum: Mechanical Engineering
-

Heat absorbed by an ideal gas in a cycle
Homework Statement Homework Equations and the attempt at a solution:[/B] AC is adiabatic and AB is isothermal. Heat absorbed during process AC = 0 (adiabatic). Heat absorbed during process CB = C_p \triangle T=-\frac{\gamma}{\gamma -1} (P_2V_3 - P_2V_2) Heat absorbed during process BA =...- Isomorphism
- Thread
- Cycle First law of thermodynamics Gas Heat Heat absorbed Ideal gas
- Replies: 1
- Forum: Introductory Physics Homework Help
-
C
Graduate Specific heat ratio of gas mixture
I am doing some multi-fluid hydrodynamic modelling and I have a quick question. I think I know the answer, but I am not convinced. One of the things that I need to know is the specific heat ratio, ##\gamma##, for the gas and my question is, how does one calculate this from the values of each...- colinjohnstoe
- Thread
- Gas Heat Mixture Ratio Specific Specific heat
- Replies: 6
- Forum: Thermodynamics
-

Calculate Heat of Combustion of Graphite
Homework Statement The heat of combustion of graphite and that of CO is 394kJ/mol and 283 kJ/mol, respectively. When 12g of graphite is combusted incompletely, the same volume of CO and CO2 are generated. Calculate the heat of generated by this combustion. Choose the closet value.(kJ) A.197...- Hamal_Arietis
- Thread
- Combustion Graphite Heat Heat of combustion
- Replies: 7
- Forum: Biology and Chemistry Homework Help
-
M
Undergrad Temperature of Plexiglass due to laser strike
Hello, Any hep is appreciated. In the lab we want to use a 0.3mm sheet of plexi to reduce the intensity of the UV KrF laser. We want to determine the temperature of the plexi sheet after the laser beam strikes it. We have all the information about the laser... Is there a specific formula...- MelissaM
- Thread
- Heat Laser Laser beam Tempeature Temperature
- Replies: 24
- Forum: Thermodynamics
-

Calculating Heat Exchange Requirements for Ethanol Condenser
Homework Statement Hi, I need someone to help me with this question. I tried to find the answer for two weeks I could not finde it. Anyone with the required experience can help! I will be so grateful. Question: A shell-and-tube heat exchanger is to act as a condenser: saturated ethyl alcohol...- OMANII_93
- Thread
- Condenser Ethanol Exchange Heat Heat exchange
- Replies: 9
- Forum: Engineering and Comp Sci Homework Help
-
T
Thermal Physics (Specific Heat Capacity)
Homework Statement Body X whose temperature is 0 °C is brought into thermal contact with body Y of equal mass and temperature 100 °C. The only exchanges of heat that take place are between X and Y. The specific heat capacity of X is greater than that of Y. Which statement about the final...- Tikiboom1
- Thread
- Capacity Final temperature Heat Heat capacity Physics Specific heat capacity Thermal Thermal physics
- Replies: 1
- Forum: Introductory Physics Homework Help
-
A
Consider the following processes: I. Energy flows as heat....
Homework Statement Consider the following processes: I. Energy flows as heat from a hot object to a colder object II. Work is done on a system and an equivalent amount of energy is rejected as heat by the system III. Energy is absorbed as heat by a system and an equivalent amount of work is...- Any Help
- Thread
- Energy Heat
- Replies: 5
- Forum: Introductory Physics Homework Help
-

Vapour pressure vs saturation pressure; too much confusion
It was only the psychrometry came; I read about vapour pressure is equal to the saturation pressure at 100% relative humidity. While before even in the textbooks both terms are used frequently as same. I fully understood what saturation pressure is; learned during phase change phenomenon of...- Ravi Singh choudhary
- Thread
- Confusion Heat Humidity Pressure Saturation Thermodynamics Vapour Vapour pressure
- Replies: 3
- Forum: Mechanical Engineering
-
F
High School Trivial Question - rate of heat loss from hot drink
Hello people of PhysicsForum Apologies in advance if my prefix selection is inaccurate and for my absolute physics noobness. I know no physics jargon so I'm sure my question will be phrased in the same way a 10 year old might. So here is my silly, trivial question: First of all, remove all...- Francescos123
- Thread
- Heat Heat loss Hot Loss Rate
- Replies: 13
- Forum: Thermodynamics
-
K
Efficient way to convert kinetic energy to heat
I am trying to find the most efficient way to convert kinetic energy to heat. The first thing I know is friction, however friction cause wearing for long term use. The second is convert to electric energy(dynamo) and then convert to heat using electric coil, however I think that has low...- kevin_tee
- Thread
- Convert Energy Heat Kinetic Kinetic energy
- Replies: 13
- Forum: Mechanical Engineering
-

How to Calculate Potential Energy and Latent Heat in Physics?
I have two homework questions that I am desperate for some help on. I've posted them on our class discussion page, and I'm on my last attempt. We get three attempts on our homework. The first question deals with SPE and the second deals with latent heat. For Question 1: 1. Homework Statement...- Ashleykins
- Thread
- Heat Latent heat
- Replies: 9
- Forum: Introductory Physics Homework Help